Alprolix Dosage
Generic name: EFTRENONACOG ALFA 500[iU] in 5mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on May 16, 2024.
For intravenous use after reconstitution only
Dose
- Dose and duration of treatment depend on the severity of the Factor IX deficiency, the location and extent of bleeding, the individual patient's pharmacokinetic profile, and/or the patient's clinical condition.
- Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses. Base the dose and frequency of ALPROLIX on the individual clinical response.
- More frequent or higher doses may be needed in children <12 years of age, especially in children <6 years of age. For patients 12 years of age or older, age-based dose adjustment is not usually required.
- In addition to the nominal (target) potency, the actual Factor IX potency in international units (IU), determined by the quality control laboratory at product release, is stated on each ALPROLIX vial label. ALPROLIX potency is assigned using a validated in vitro, activated partial thromboplastin time (aPTT)–based, one-stage clotting assay calibrated against the World Health Organization (WHO) international standard for Factor IX concentrates.
- Factor IX activity measurements in the clinical laboratory may be affected by the type of aPTT reagent or laboratory standard used.
On average, one IU of ALPROLIX per kg body weight increases the circulating level of Factor IX by approximately 1% (IU/dL) in adults and children ≥6 years of age and by 0.6% (IU/dL) in children under 6 years of age. Estimate the required dose or the expected in vivo peak increase in Factor IX level expressed as IU/dL (or % of normal) using the following formulas:
| IU/dL (or % of normal) = (Total Dose [IU]/Body Weight [kg]) × Recovery (IU/dL per IU/kg) OR Dose (IU) = Body Weight (kg) × Desired Factor IX Rise (IU/dL or, % of normal) × Reciprocal of Recovery (IU/kg per IU/dL) |
- Consider determining the patient's in vivo recovery (in IU/dL per IU/kg) prior to elective major surgery and verify that the target Factor IX level has been achieved prior to major surgery and for major bleeds.
On-demand Treatment and Control of Bleeding Episodes
ALPROLIX dosing for on-demand treatment and control of bleeding episodes is provided in Table 1.
| Type of Bleeding | Circulating Factor IX Level Required (IU/dL or % of normal) | Dosing Interval (hours) |
|---|---|---|
| Minor and Moderate For example: Uncomplicated hemarthroses, superficial muscle (except iliopsoas) without neurovascular compromise, superficial soft tissue, mucous membranes |
30–60 | Repeat every 48 hours if there is further evidence of bleeding. |
| Major For example: Iliopsoas and deep muscle with neurovascular injury, or substantial blood loss; Pharyngeal, retropharyngeal, retroperitoneal, CNS |
80–100 | Consider a repeat dose after 6–10 hours and then every 24 hours for the first 3 days. Due to the long half-life of ALPROLIX, the dose may be reduced and frequency of dosing may be extended after day 3 to every 48 hours or longer until bleeding stops and healing is achieved. |
Perioperative Management of Bleeding
ALPROLIX dosing for perioperative management is provided in Table 2.
| Type of Surgery | Circulating Factor IX Level Required (IU/dL or % of normal) | Dosing Interval (hours) |
|---|---|---|
| Minor (including uncomplicated dental extraction) |
50–80 | A single infusion may be sufficient. Repeat as needed after 24–48 hours until bleeding stops and healing is achieved. |
| Major | 60–100 (initial level) |
Consider a repeat dose after 6–10 hours and then every 24 hours for the first 3 days. Due to the long half-life of ALPROLIX, the dose may be reduced and frequency of dosing in the post-surgical setting may be extended after day 3 to every 48 hours or longer until bleeding stops and healing is achieved. |
Routine Prophylaxis
- The recommended starting regimens for adults and adolescents ≥12 years of age are either 50 IU/kg once weekly, or 100 IU/kg once every 10 days.
- For children <12 years of age, start with 60 IU/kg once weekly.
- Adjust dosing regimen based on individual response. More frequent or higher doses may be needed in children <12 years of age, especially in children <6 years of age.
Reconstitution
- Use aseptic technique (clean and germ-free) and a flat work surface during the reconstitution procedure.
- Allow the vial of ALPROLIX, containing the white to off-white lyophilized powder and the prefilled diluent syringe to reach room temperature before use.
- Remove the plastic cap from the vial and wipe the rubber stopper of the vial with an alcohol wipe. Allow the rubber stopper to dry.
-
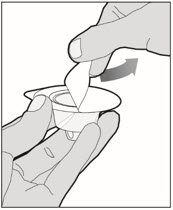
Completely remove the backing from the vial adapter package by peeling back the lid. Do not remove the vial adapter from the package or touch the inside of the package of the adapter.
-
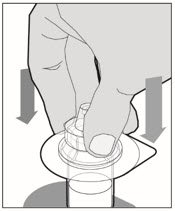
Place the vial on a flat surface and use one hand to hold the vial steady. Use the other hand to place the vial adapter over the vial. Place the adapter spike directly above the center of the rubber stopper and push the adapter straight down until the spike punctures the center of the vial stopper and is fully inserted.
-
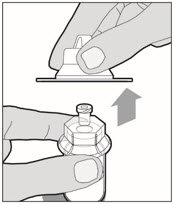
Lift the package cover away from the vial adapter and discard the cover.
-
Hold the plunger rod at the circular disk. Place the tip of the plunger rod into the end of the syringe. Turn clockwise until it is securely attached. Only use the diluent syringe provided in the ALPROLIX package.
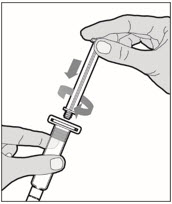
- With one hand, hold the diluent syringe by the ridged part directly under the cap, with the cap pointing up. Do not use if the cap has been removed or is not securely attached.
- With your other hand, grasp the cap and bend it at a 90° angle until it snaps off. After the cap snaps off, you will see the glass tip of the syringe. Do not touch the glass tip of the syringe or the inside of the cap.
- With the vial sitting on a flat surface, insert the tip of the syringe into the adapter opening. Turn the syringe clockwise until it is securely attached to the adapter.
- Slowly depress the plunger rod to inject all of the diluent into the vial. The plunger rod may rise slightly after this process. This is normal.
- With the syringe still connected to the adapter, gently swirl the vial until the product is completely dissolved. The final solution should be clear to slightly opalescent and colorless. Do not shake. Do not use the reconstituted ALPROLIX if it contains visible particles or is cloudy.
- Make sure the plunger rod is completely depressed. Turn the vial upside-down. Slowly pull on the plunger rod to draw the solution into the syringe. Be careful not to pull the plunger rod completely out of the syringe.
- Gently unscrew the syringe from the vial adapter and dispose of the vial with the adapter still attached. Do not touch the syringe tip or the inside of the cap.
- Use the reconstituted ALPROLIX as soon as possible, but no later than 3 hours after reconstitution. Protect from direct sunlight. Do not refrigerate after reconstitution.
To combine two or more vials of ALPROLIX, after step 12 above, follow these pooling steps:
- Remove the diluent syringe from the vial adapter by turning it counterclockwise until it is completely detached. Do not detach the diluent syringe or the large luer lock syringe until ready to attach the large luer lock syringe to the next vial (with vial adapter attached).
- Leave the vial adapter attached to the vial, as it is needed for attaching a large luer lock syringe.
- Attach a separate, large luer lock syringe by turning clockwise until it is securely in place.
- Slowly pull on the plunger rod to draw the solution into the syringe.
- Repeat this pooling procedure with each vial necessary to obtain the required dose. Once you have pooled the required dose, proceed to administration using the large luer lock syringe.
Administration
For intravenous injection only
- Inspect the reconstituted ALPROLIX solution visually for particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not administer reconstituted ALPROLIX in the same tubing or container with other medications.
Administration Steps:
- Attach the syringe to the connector end of the infusion set tubing by turning clockwise until it is securely in place.
- Depress the plunger until all air is removed from the syringe and ALPROLIX has reached the end of the infusion set tubing. Do not push ALPROLIX through the needle.
- Remove the protective needle cover from the infusion set tubing.
- Perform intravenous bolus infusion. The rate of administration should be determined by the patient's comfort level, and no faster than 10 mL per minute.
After infusing ALPROLIX, remove and properly discard the infusion set.
More about Alprolix (coagulation factor ix)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
- Alprolix drug information
- Alprolix (Factor ix fc fusion protein recombinant Intravenous) (Advanced Reading)
Other brands
Idelvion, BeneFix, Rebinyn, Alphanine SD, ... +3 more
Professional resources
Other brands
Idelvion, BeneFix, Rebinyn, Alphanine SD, ... +3 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.