Hexabrix: Package Insert / Prescribing Info
Package insert / product label
Generic name: ioxaglate meglumine and ioxaglate sodium
Dosage form: injection
Drug class: Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
Hexabrix Description
HEXABRIX is a sterile, non-pyrogenic, aqueous solution intended for use as a diagnostic radiopaque medium. HEXABRIX contains 39.3% w/v N-(2-hydroxyethyl)-2,4,6-triiodo-5-[2-[2,4,6-triiodo-3-(N-methylacetamido)-5-(methylcarbamoyl) benzamido] acetamido]-isophthalamic acid, compounded with 1-deoxy-1-(methylamino)-D-glucitol (1:1) and 19.6% w/v sodium N-(2-hydroxyethyl)-2,4,6 triiodo-5-[2-[2,4,6-triiodo-3-(N-methylacetamido)-5-(methylcarbamoyl) benzamido] acetamido]-isophthalamate.
The two salts have the following structural formulae:
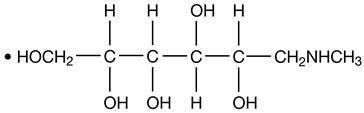
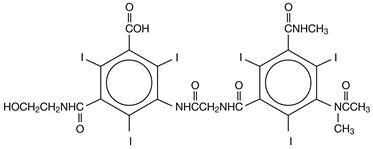
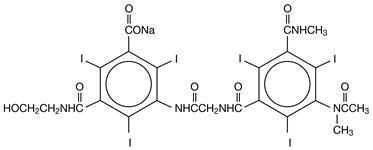
†Licensed by Guerbet, S.A.
Registered U.S. Patent and Trademark Office
Each milliliter contains 393 mg of ioxaglate meglumine, 196 mg of ioxaglate sodium and 0.10 mg edetate calcium disodium as a stabilizer. The solution contains 3.48 mg (0.15 mEq) sodium in each milliliter and provides 32% (320 mg/mL) organically bound iodine.
Solutions of ioxaglate (HEXABRIX) provide six iodine atoms for each two dissociated ions. HEXABRIX is an ionic contrast agent. HEXABRIX has an osmolarity of approximately 460 mOsmol/L, an osmolality of approximately 600 mOsmol/kg of water and is, therefore, hypertonic under conditions of use.
HEXABRIX has a viscosity (cps) of 15.7 at 20°C and 7.5 at 37°C. The pH has been adjusted to 6.0 to 7.6 with meglumine, sodium hydroxide or ioxaglic acid.
HEXABRIX is a clear, colorless to pale yellow solution containing no undissolved solids. Crystallization does not occur at normal room temperatures. It is supplied in containers from which the air has been displaced by nitrogen.
Hexabrix - Clinical Pharmacology
Intravascular injection of a radiopaque diagnostic agent opacifies those vessels in the path of the flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs.
Following intravascular injection, HEXABRIX is rapidly transported through the circulatory system to the kidneys and is excreted unchanged in the urine. The pharmacokinetics of intravascularly administered radiopaque contrast media are usually best described by a two compartment model with a rapid alpha phase for drug distribution and a slower beta phase for drug elimination. In 10 patients with normal renal function, the alpha and beta half-lives of HEXABRIX were 12 (4-17) and 92 (61-140) minutes, respectively. Following the intravenous administration of 50 mL of HEXABRIX in 10 normal volunteers, the mean peak plasma concentration occurred at two (1-3) minutes, reaching a concentration of 2.1 (1.8-2.8) mg/mL. Approximately 50 (42-67) percent of the intravenously administered dose was recovered in the urine at two hours, and 90 (68-105) percent was recovered at 24 hours.
The joint spaces as well as the uterus and fallopian tubes may be visualized by the direct injection of the contrast medium into the region to be studied.
Injectable iodinated contrast agents are excreted either through the kidneys or through the liver. These two excretory pathways are not mutually exclusive, but the main route of excretion seems to be related to the affinity of the contrast medium for serum albumin. Ioxaglate salts are poorly bound to serum albumin, and are excreted mainly through the kidneys.
The liver and small intestine provide the major alternate route of excretion. In patients with severe renal impairment, the excretion of this contrast medium through the gallbladder and into the small intestine sharply increases.
Ioxaglate salts cross the placental barrier in humans and are excreted unchanged in human milk.
CT SCANNING OF THE HEAD
When used for contrast enhancement in computed tomographic head imaging, the degree of enhancement is directly related to the amount of iodine administered. Rapid injection of the entire dose yields peak blood iodine concentrations immediately following the injection, which falls rapidly over the next five to ten minutes as a result of dilution in the vascular and extravascular fluid compartments. Equilibration is reached in about ten minutes and thereafter the fall in iodine plasma concentration becomes exponential.
In brain scanning, contrast media do not accumulate in normal brain tissue due to the blood brain barrier (BBB). The increase in x-ray attenuation usually seen in normal tissue following contrast medium injection is due to the presence of the contrast medium in the blood pool. Disruption in the BBB, such as occurs in malignant tumors of the brain, allows accumulation of contrast medium within the interstitial tumor tissue; adjacent normal brain tissue does not contain the contrast medium. Maximum contrast enhancement frequently occurs after peak blood iodine levels are reached. A delay in maximum contrast enhancement can occur depending on the peak iodine level achieved and the cell type of the lesion. This lag in enhancement is probably associated with the accumulation of the contrast medium within the lesion and outside the blood pool.
The image enhancement of non-tumor lesions, such as arteriovenous malformations and aneurysms, is dependent on the iodine content of the circulating blood pool.
CT SCANNING OF THE BODY
HEXABRIX may also be used for enhancement of computed tomographic scans performed for detection and evaluation of lesions in the liver, pancreas, kidneys, abdominal aorta, mediastinum, abdominal cavity and retroperitoneal space.
In non-neural tissues (during computed tomography of the body), HEXABRIX diffuses rapidly from the vascular to the extra-vascular space. Increase in x-ray absorption is related to blood flow, concentration of the contrast medium and extraction of the contrast medium by interstitial tissue since no barrier exists; contrast enhancement is thus due to the relative differences in extra-vascular diffusion between normal and abnormal tissue, a situation quite different than that in the brain.
The pharmacokinetics of HEXABRIX in normal and abnormal tissues has been shown to be variable.
Enhancement of CT with HEXABRIX may be of benefit in establishing diagnoses of certain lesions in some sites with greater assurance than is possible with unenhanced CT and in supplying additional features of the lesions. In other cases, the contrast medium may allow visualization of lesions not seen with CT alone or may help to define suspicious lesions seen with unenhanced CT.
Contrast enhancement appears to be greatest within the 30-90 seconds after bolus administration of the contrast agent, and after intra-arterial rather than intravenous administration. Therefore, the use of a continuous scanning technique (a series of two to three second scans beginning at the injection — dynamic CT scanning) may improve enhancement and diagnostic assessment of tumors and other lesions such as an abscess, occasionally revealing more extensive disease.
Because unenhanced scanning may provide adequate information in the individual patient, the decision to employ contrast enhancement, which is associated with additional risk and increased radiation exposure, should be based upon a careful evaluation of clinical, other radiological and unenhanced CT findings.
Indications and Usage for Hexabrix
HEXABRIX is indicated for use in pediatric angiocardiography, selective coronary arteriography with or without left ventriculography, peripheral arteriography, aortography, selective visceral arteriography, cerebral angiography, intra-arterial digital subtraction angiography, intravenous digital subtraction angiography, peripheral venography (phlebography), excretory urography, contrast enhancement of computed tomographic head imaging and body imaging, arthrography and hysterosalpingography.
Contraindications
HEXABRIX is contraindicated for use in myelography. Refer to PRECAUTIONS, General, concerning hypersensitivity. Hysterosalpingography should not be performed during the menstrual period; in pregnant patients; in patients with known infection in any portion of the genital tract; or in patients in whom cervical conization or curettage has been performed within 30 days. Arthrography should not be performed if infection is present in or near the joint.
WARNINGS
SEVERE ADVERSE EVENTS — INADVERTENT INTRATHECAL ADMINISTRATION: Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that this drug product is not administered intrathecally.
Ionic iodinated contrast media inhibit blood coagulation, in vitro, more than nonionic contrast media. Nonetheless, it is prudent to avoid prolonged contact of blood with syringes containing ionic contrast media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/ or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
Serious or fatal reactions have been associated with the administration of iodine containing radiopaque media. It is of utmost importance to be completely prepared to treat any contrast medium reaction.
As with any contrast medium, serious neurologic sequelae, including permanent paralysis, can occur following cerebral arteriography, selective spinal arteriography and arteriography of vessels supplying the spinal cord. The injection of a contrast medium should never be made following the administration of vasopressors since they strongly potentiate neurologic effects.
In patients with subarachnoid hemorrhage, a rare association between contrast administration and clinical deterioration, including convulsions and death, has been reported. Therefore, administration of intravascular iodinated contrast media in these patients should be undertaken with caution.
A definite risk exists in the use of intravascular contrast agents in patients who are known to have multiple myeloma. In such instances anuria has developed resulting in progressive uremia, renal failure and eventually death. Although neither the contrast agent nor dehydration has separately proved to be the cause of anuria in myeloma, it has been speculated that the combination of both may be causative factors. The risk in myelomatous patients is not a contraindication to the procedure; however, partial dehydration in the preparation of these patients for the examination is not recommended since this may predispose to precipitation of myeloma protein in the renal tubules. No form of therapy, including dialysis, has been successful in reversing the effect. Myeloma, which occurs most commonly in persons over 40, should be considered before instituting intravascular administration of contrast agents.
Administration of radiopaque materials to patients known or suspected to have pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure, and measures for treatment of a hypertensive crisis should be available.
Since intravascular administration of contrast media may promote sickling in individuals who are homozygous for sickle cell disease, fluid restriction is not advised.
In patients with advanced renal disease, iodinated contrast media should be used with caution and only when the need for the examination dictates, since excretion of the medium may be impaired. Patients with combined renal and hepatic disease, those with severe hypertension or congestive heart failure and recent renal transplant recipients present an additional risk.
Renal failure has been reported in patients with liver dysfunction who were given an oral cholecystographic agent followed by an intravascular iodinated radiopaque agent and also in patients with occult renal disease, notably diabetics and hypertensives. In these classes of patients there should be no fluid restriction and every attempt made to maintain normal hydration, prior to contrast medium administration, since dehydration is the single most important factor influencing further renal impairment.
Caution should be exercised in performing contrast medium studies in patients with endotoxemia and/or those with elevated body temperatures.
Reports of thyroid storm occurring following the intravascular use of iodinated radiopaque agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule, suggest that this additional risk be evaluated in such patients before use of this drug. Iodine containing contrast agents may alter the results of thyroid function tests which depend on iodine estimation, e.g., PBI, and may also affect results of radioactive iodine uptake studies. Such tests, if indicated, should be performed prior to the administration of this preparation.
Precautions
General
Diagnostic procedures which involve the use of iodinated intravascular contrast agents should be carried out under the direction of personnel skilled and experienced in the particular procedure to be performed. All procedures utilizing contrast media carry a definite risk of producing adverse reactions. While most reactions are minor, life-threatening and fatal reactions may occur without warning, and this risk must be weighed against the benefit of the procedure. A fully equipped emergency cart, or equivalent supplies and equipment, and personnel competent in recognizing and treating adverse reactions of all types should always be available. If a serious reaction should occur, immediately discontinue administration. Since severe delayed reactions have been known to occur, emergency facilities and competent personnel should be available for at least 30 to 60 minutes after administration. (See ADVERSE REACTIONS, General.)
Preparatory dehydration is dangerous and may contribute to acute renal failure in infants, young children, the elderly, patients with pre-existing renal insufficiency, patients with multiple myeloma, patients with advanced vascular disease and diabetic patients.
Acute renal failure has been reported in diabetic patients with diabetic nephropathy and in susceptible nondiabetic patients (often elderly with pre-existing renal disease) following the administration of iodinated contrast agents. Therefore, careful consideration of the potential risks should be given before performing this radiographic procedure in these patients.
Severe reactions to contrast media often resemble allergic responses. This has prompted the use of several provocative pretesting methods, none of which can be relied on to predict severe reactions. No conclusive relationship between severe reactions and antigen-antibody reactions or other manifestations of allergy has been established. The possibility of an idiosyncratic reaction in patients who have previously received a contrast medium without ill effect should always be considered. Prior to the injection of any contrast medium, the patient should be questioned to obtain a medical history with emphasis on allergy and hypersensitivity. A positive history of bronchial asthma or allergy (including food), a family history of allergy, or a previous reaction or hypersensitivity to a contrast agent may imply a greater than usual risk. Such a history may be more accurate than pre-testing in predicting the potential for reaction, although not necessarily the severity or type of reaction in the individual case. A positive history of this type does not arbitrarily contraindicate the use of a contrast agent, when a diagnostic procedure is thought essential, but does call for caution. (See ADVERSE REACTIONS, General.)
Prophylactic therapy including corticosteroids and antihistamines should be considered for patients who present with a strong allergic history, a previous reaction to a contrast medium, or a positive pre-test since in these patients the incidence of reaction is two to three times that of the general population. Adequate doses of corticosteroids should be started early enough prior to contrast medium injection to be effective and should continue through the time of injection and for 24 hours after injection. Antihistamines should be administered within 30 minutes of the contrast medium injection. Recent reports indicate that such pre-treatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity. A separate syringe should be used for these injections.
General anesthesia may be indicated in the performance of some procedures in selected patients; however, a higher incidence of adverse reactions has been reported in these patients, and may be attributable to the inability of the patient to identify untoward symptoms or to the hypotensive effect of anesthesia which can prolong the circulation time and increase the duration of contact of the contrast agent.
Angiography should be avoided whenever possible in patients with homocystinuria because of the risk of inducing thrombosis and embolism.
Information for Patients: Patients receiving iodinated intravascular contrast agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease or known thyroid disease. (See WARNINGS).
- Inform your physician if you are allergic to any drugs, food or if you had any reactions to previous injections of dyes used for x-ray procedures. (See PRECAUTIONS, General).
- Inform your physician about any other medications you are currently taking including non-prescription drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term animal studies have been performed to evaluate carcinogenic potential. However, animal studies suggest that this drug is not mutagenic and does not affect fertility in males or females.
Pregnancy Category B:
Reproduction studies have been performed in rats, and rabbits at doses up to two times the maximum adult human dose and have revealed no evidence of impaired fertility or harm to the fetus due to HEXABRIX. There are however no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers:
Ioxaglate salts are excreted unchanged in human milk. Because of the potential for adverse effects in nursing infants, bottle feedings should be substituted for breast feedings for 24 hours following the administration of this drug.
Pediatric Use:
Safety and effectiveness in children have been established in pediatric angiocardiography and intravenous excretory urography. Data have not been submitted to support the safety and effectiveness of HEXABRIX in any other indication.
(Precautions for specific procedures receive comment under that procedure.)
Adverse Reactions/Side Effects
General
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physio-chemical properties of the contrast media, the dose and the speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the dose injected, the speed of injection, the mode of injection and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
NOTE: Not all of the following adverse reactions have been reported with HEXABRIX. Because HEXABRIX is an iodinated intravascular contrast agent, all of the side effects and toxicity associated with agents of this class are theoretically possible, and this should be borne in mind when HEXABRIX is administered.
Severe, life-threatening anaphylactoid reactions, mostly of cardiovascular origin, have occurred following the administration of HEXABRIX as well as other iodine-containing contrast agents. Most deaths occur during injection or 5 to 10 minutes later; the main feature being cardiac arrest with cardiovascular disease as the main aggravating factor. Isolated reports of hypotensive collapse and shock are found in the literature. Based upon clinical literature, reported deaths from the administration of conventional iodinated contrast agents range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent).
Regardless of the contrast agent employed, the overall estimated incidence of serious adverse reactions is higher with coronary arteriography than with other procedures. Cardiac decompensation, serious arrhythmias, or myocardial ischemia or infarction may occur during coronary arteriography and left ventriculography.
The most frequent adverse reactions are nausea, vomiting, facial flush and a feeling of body warmth. These are usually of brief duration. In double-blind clinical trials, HEXABRIX produced less discomfort upon injection (pain and heat) when compared to various other contrast agents. Other reactions include the following:
Hypersensitivity reactions: Dermal manifestations of urticaria with or without pruritus, erythema and maculopapular rash. Dry mouth. Sweating. Conjunctival symptoms. Facial, peripheral and angioneurotic edema. Symptoms related to the respiratory system include sneezing, nasal stuffiness, coughing, choking, dyspnea, chest tightness and wheezing, which may be initial manifestation of more severe and infrequent reactions including asthmatic attack, laryngospasm and bronchospasm with or without edema, pulmonary edema, apnea and cyanosis. Rarely, these allergic-type reactions can progress into anaphylaxis with loss of consciousness, coma, severe cardiovascular disturbances, and death.
Cardiovascular reactions: Generalized vasodilation, flushing and venospasm. Occasionally, thrombosis or rarely, thrombophlebitis. Extremely rare cases of disseminated intravascular coagulation resulting in death have been reported. Severe cardiovascular responses include rare cases of hypotensive shock, coronary insufficiency, cardiac arrhythmia, fibrillation and arrest. These severe reactions are usually reversible with prompt and appropriate management; however, fatalities have occurred.
Technique reactions: Extravasation with burning pain, hematomas, ecchymosis and tissue necrosis, vascular constriction due to injection rate, thrombosis and thrombophlebitis.
Neurological reactions: Spasm, convulsions, aphasia, syncope, paresis, paralysis resulting from spinal cord injury and pathology associated with the syndrome of transverse myelitis, visual field losses which are usually transient but may be permanent, coma and death.
Endocrine reactions: Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration to adult and pediatric patients, including infants. Some patients were treated for hypothyroidism.
Other reactions: Headache, trembling, shaking, chills without fever, hyperthermia and lightheadedness. Temporary renal shutdown or other nephropathy.
(Adverse reactions to specific procedures receive comment under that procedure.)
Related/similar drugs
Overdosage
Overdosage may occur. The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular systems. The symptoms may include cyanosis, bradycardia, acidosis, pulmonary hemorrhage, convulsions, coma and cardiac arrest. Treatment of an overdose is directed toward the support of all vital functions and prompt institution of symptomatic therapy.
Ioxaglate salts are dialyzable.
The intravenous LD50 values of HEXABRIX (in grams of iodine/kilogram body weight) were 11.2 g/kg in mice, >8 g/kg in rats, >6.4 g/kg in rabbits and >10.2 g/kg in dogs.
Hexabrix Dosage and Administration
It is advisable that HEXABRIX be at or close to body temperature when injected.
The patient should be instructed to omit the meal that precedes the examination. Appropriate premedication, which may include a barbiturate, tranquilizer or analgesic drug, may be administered prior to the examination.
A preliminary film is recommended to check the position of the patient and the x-ray exposure factors prior to the injection of the contrast medium.
If during administration a minor reaction occurs the injection should be slowed or stopped until the reaction has subsided. If a major reaction occurs the injection should be discontinued immediately.
Under no circumstances should other drugs be administered concomitantly in the same syringe or IV administration set because of a potential for chemical incompatibility.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
PEDIATRIC ANGIOCARDIOGRAPHY
HEXABRIX may be administered by catheter injection into the chambers of the heart or associated large blood vessels. Rapid injection is essential and satisfactory results usually require injection of the total dosage in 1-2 seconds.
Precautions
In addition to the general precautions previously described, it is advisable to monitor for ECG and vital signs changes throughout the procedure.
When large individual doses are administered sufficient time should be allowed for any observed changes to return to or near baseline prior to making the next injection.
Caution should be used when making right heart injections in patients with pulmonary hypertension or incipient heart failure since this may lead to increased right side pressures with subsequent bradycardia and systemic hypotension. Patients with pulmonary disease present additional risks.
Caution is advised in cyanotic infants since apnea, bradycardia, other arrhythmias and a tendency to acidosis are more likely to occur.
Since infants are more likely to respond with convulsions than are adults, the amount of total dosage is of particular importance. Repeated injections are hazardous in infants weighing less than 7 kg, particularly when these infants have pre-existing compromised right heart function or obliterated pulmonary vascular beds.
Adverse Reactions
In addition to the adverse reactions previously listed, this procedure has been complicated by intramural injection with marked adverse effects on cardiac function.
Usual Dosage
The volume of individual doses should be determined by the size of the structure to be visualized and the anticipated degree of hemodilution at the site of injection. Valvular competence should also be taken into consideration.
Older Children: Catheter angiocardiography usually requires single doses of 30-45 mL of HEXABRIX.
Infants and Young Children: The recommended single dose of HEXABRIX is about 1.5 mL/kg (range 1 mL/kg to 2 mL/kg). In addition, small test volumes of about 2 mL may be used for catheter placement.
The usual total dose of HEXABRIX per procedure, which includes diagnostic and test doses is about 4 mL/kg. This dosage may be as small as 1.5 mL/kg and should not normally exceed 5 mL/kg.
SELECTIVE CORONARY ARTERIOGRAPHY WITH OR WITHOUT LEFT VENTRICULOGRAPHY
Precautions
During the administration of large doses of HEXABRIX, continuous monitoring of vital signs is desirable. Caution is advised in the administration of large volumes to patients with incipient heart failure because of the possibility of aggravating the pre-existing condition. Hypotension should be corrected promptly since it may result in serious arrhythmias.
Special care regarding dosage should be observed in patients with right ventricular failure, pulmonary hypertension, or stenotic pulmonary vascular beds because of hemodynamic changes which may occur after injection into the right heart outflow tract.
Adverse Reactions
Patients may have clinically insignificant ECG changes during the procedure. The following adverse effects have occurred in conjunction with the administration of iodinated intravascular contrast agents for this procedure: hypotension, shock, anginal pain, myocardial infarction, cardiac arrhythmias (bradycardia, ventricular tachycardia, ventricular fibrillation) and cardiac arrest. Fatalities have been reported.
Complications to the procedure include dissection of coronary arteries, dislodgement of atheromatous plaques, perforation, hemorrhage and thrombosis.
Usual Dosage
The usual adult dose for left coronary arteriography is 8 mL (range 2-14 mL) and for right coronary arteriography is 5 mL (range 1-10 mL). The doses may be repeated as necessary; doses up to a total of 150 mL have been given. For left ventriculography, the usual adult dose in a single injection is 45 mL (range 35-45 mL) and repeated as necessary. The total dose for combined selective coronary arteriography and left ventriculography should not exceed 250 mL.
PERIPHERAL ARTERIOGRAPHY
HEXABRIX may be injected to visualize the peripheral arterial circulation. Arteriograms of the upper and lower extremities may be obtained by any of the established techniques.
Patient Preparation
The procedure is normally performed with local anesthesia. Rarely, general anesthesia may be required. (See PRECAUTIONS, General.)
A preliminary radiograph is usually made prior to the injection of the contrast agent.
Precautions
In addition to the general precautions previously described, moderate decreases in blood pressure occur frequently with intra-arterial (brachial) injections. This change is usually transient and requires no treatment, however, the blood pressure should be monitored for approximately ten minutes following injection.
Extreme caution during injection of the contrast agent is necessary to avoid extravasation and fluoroscopy is recommended. This is especially important in patients with severe arterial disease.
Adverse Reactions
In addition to the general adverse reactions previously described, hemorrhage and thrombosis have occurred at the puncture site of the percutaneous injection. Brachial plexus injury has been reported following axillary artery injection.
Usual Dosage
The single adult dose for aorto-iliac runoff studies is 45 mL (range 20-80 mL). The single adult dose for the common iliac, the external iliac and the femoral arteries is 30 mL (range 10-50 mL). These doses may be repeated as necessary. For the upper limb, the usual single adult dose is 20 mL (range 15-30 mL), repeated as necessary. The total procedural dose should not exceed 250 mL.
AORTOGRAPHY AND SELECTIVE VISCERAL ARTERIOGRAPHY
HEXABRIX may be used to visualize the aorta and its major abdominal branches.
CEREBRAL ANGIOGRAPHY
HEXABRIX may be used to visualize the cerebral vasculature by any of the accepted techniques.
Patient Preparation
Cerebral angiography is normally performed with local or general anesthesia. (See PRECAUTIONS, General.)
Precautions
In addition to the general precautions previously described, cerebral angiography should be performed with special caution in patients with advanced arteriosclerosis, severe hypertension, cardiac decompensation, senility, recent cerebral thrombosis or embolism, and migraine.
Adverse Reactions
The major causes of cerebral arteriographic adverse reactions appear to be repeated injections of the contrast material, administration of doses higher than those recommended, the presence of occlusive atherosclerotic vascular disease and the method and technique of injection.
Adverse reactions are normally mild and transient. A feeling of warmth in the face and neck is frequently experienced. Infrequently, a more severe burning discomfort is observed. Transient visual hallucinations have been reported.
Serious neurological reactions that have been associated with cerebral angiography and not listed under Adverse Reactions, General, include stroke, amnesia and respiratory difficulties.
Visual field defects with anopsia and reversible neurological deficit lasting from 24 hours to 48 hours have been reported. Confusion, disorientation with hallucination, and absence of vision sometimes lasting for one week have also been reported.
Cardiovascular reactions that may occur with some frequency are bradycardia and either an increase or decrease in systemic blood pressure. The blood pressure change is transient and usually requires no treatment.
Usual Dosage
The usual dosage employed varies with the site and method of injection and the age and condition of the patient. In adults, cerebral angiography is usually performed by a selective injection of 9 mL (range 6-12mL) for the common carotid arteries and 8 mL (range 5-12 mL) for the vertebral arteries. Additional injections may be made as indicated. When aortic arch injections (four vessel studies) are performed in conjunction with cerebral angiography, the usual dose is 40 mL (range 30-50 mL). Other dosages may be employed for more selective injections, depending upon the vessel injected. The total dose per procedure should not exceed 150 mL.
INTRA-ARTERIAL DIGITAL SUBTRACTION ANGIOGRAPHY (IA-DSA)
Intra-arterial digital subtraction angiography (IA-DSA) is a radiographic modality which produces arterial images similar to conventional film-screen systems following arterial injection. The advantages include: the use of less contrast medium; the use of lower iodine concentrations; a decreased need for selective arterial catheterization; and a shortened examination time.
Patient Preparation
No special patient preparation is required for IA-DSA. However, it is advisable to insure that patients are well hydrated prior to examination.
Precautions
In addition to the general precautions described, the risks associated with IA-DSA are those usually attendant with catheter procedures. Following the procedure, gentle pressure hemostasis is required, followed by observation and immobilization of the limb for several hours to prevent hemorrhage from the site of arterial puncture.
Patient motion, including respiration and swallowing, can result in misregistration leading to image degradation and non-diagnostic studies.
Usual Dosage
As a general rule, the volume and concentration used for IA-DSA are about 50%, or less, of that used for conventional procedures. The actual dosage and flow rate will vary depending on the selectivity of the injection site and the area being examined.
The most versatile concentration of HEXABRIX is a 1:1 dilution with Sterile Water for Injection, U.S.P. This dilution provides 16% iodine and is isotonic.
The following suggested volumes per injection are intended only as a guide. Injections may be repeated as necessary. It is advisable to inject at rates approximately equal to the flow rate of the vessel being injected.
| Carotid Arteries | 6-10 mL |
| Vertebral Arteries | 4-8 mL |
| Aorta | 25-50 mL |
| Subclavian or Brachial Arteries | 2-10 mL |
| Major Branches of the Abdominal Aorta | 2-20 mL |
INTRAVENOUS DIGITAL SUBTRACTION ANGIOGRAPHY
Intravenous digital subtraction angiography (IV DSA) is a radiographic modality which allows dynamic imaging of the arterial system following intravenous injection of iodinated x-ray contrast media through the use of image intensification, enhancement of the iodine signal and digital processing of the image data. Temporal subtraction of the images obtained prior to and during the “first arterial pass” of the injected contrast medium yields images which are devoid of bone and soft tissue.
IV DSA is most frequently used to examine the heart, including coronary by-pass grafts; the pulmonary arteries; arteries of the brachiocephalic circulation; the aortic arch; the abdominal aorta and its major branches; the iliac arteries; and the arteries of the extremities.
Patient Preparation
No special patient preparation is required for IV DSA. However it is advisable to insure that patients are well hydrated prior to examination.
Precautions
In addition to the general precautions previously described, the risks associated with IV DSA include those usually attendant with catheter procedures and include intramural injections, vessel dissection and tissue extravasation. The potential risk is reduced when small test injections of contrast medium are made under fluoroscopic observation to insure that the catheter tip is properly positioned and, in the case of peripheral placement, that the vein is of adequate size.
Patient motion, including respiration and swallowing, can result in misregistration leading to image degradation and non-diagnostic studies.
Usual Dosage
HEXABRIX may be injected centrally, in either the superior or inferior vena cava or right atrium; or peripherally into an appropriate arm vein. For central injections, catheters may be introduced at the antecubital fossa into either the basilic or cephalic vein or at the leg into the femoral vein and advanced to the distal segment of the corresponding vena cava. For peripheral injections, the catheter is introduced at the antecubital fossa into an appropriate size arm vein. In order to reduce the potential for extravasation during peripheral injection, a catheter of approximately 20 cm in length should be employed.
Depending on the area to be imaged, the usual dose range per injection is 30-50 mL. Injections may be repeated as necessary. The total procedural dose should not exceed 250 mL.
Injection rates will vary depending on the site of catheter placement and vessel size. Central catheter injections are usually made at a rate of between 10 and 30 mL/second. Peripheral injections are usually made at a rate of between 12 and 20 mL/second. Since the injected medium can sometimes remain in the arm vein for an extended period, it may be advisable to flush the vein, immediately following injection with an appropriate volume (20-25 mL) of 5% Dextrose in water or normal saline.
PERIPHERAL VENOGRAPHY (PHLEBOGRAPHY)
HEXABRIX may be injected to visualize the peripheral venous circulation. Venograms are obtained by injection or infusion into an appropriate vein in the upper or lower extremity. Post-venography thrombophlebitis, as detected by fibrinogen I-125 uptake studies, is significantly less in patients receiving HEXABRIX when compared to conventional contrast agents.
Precautions
In addition to the general precautions previously described, special care is required when venography is performed in patients with suspected thrombosis, phlebitis, severe ischemic disease, local infection or a totally obstructed venous system.
Extreme caution during injection of contrast media is necessary to avoid extravasation and fluoroscopy is recommended. This is especially important in patients with severe arterial or venous disease.
Usual Dosage
The dose for adults will usually range from 50-100 mL per extremity of full strength (32% iodine) HEXABRIX as a single rapid injection. The dosage will vary according to the patient's size and condition and the technique employed. Smaller or larger volumes may be indicated in some cases.
Reduced concentrations to as low as 20% w/v iodine may be effectively employed. These dilute solutions may be prepared by addition of normal saline (Sodium Chloride Injection, U.S.P.), 5% Dextrose in water (D5W) or Water for Injection, U.S.P. To prepare a 20% w/v solution, dilute each milliliter of HEXABRIX with 0.6 milliliters of the diluent selected (e.g., 50 mL HEXABRIX plus 30 mL of diluent equals 80 mL of a 20% iodine concentration). The usual dose of dilute medium will range from 75-150 mL per extremity.
Following the procedure, the venous system should be flushed with any one of the diluents listed above. Massage and elevation are also helpful for clearing the contrast medium from the extremity.
EXCRETORY UROGRAPHY
Following intravenous injection, HEXABRIX is rapidly excreted by the kidneys. HEXABRIX may be visualized in the renal parenchyma one minute following bolus injection. Maximum radiographic density in the calyces and pelves occurs in most instances within 7 to 12 minutes after injection. In patients with severe renal impairment, contrast visualization may be substantially delayed.
Patient Preparation
A low residue diet the day preceding the examination and a laxative the evening before the examination may be given, unless contraindicated.
Precautions
Infants and small children should not have any fluid restrictions prior to excretory urography. (See WARNINGS and PRECAUTIONS, General concerning preparatory dehydration.)
Usual Dosage
Adults — The usual adult dose is 50 to 75 mL (0.7 to 1.0 mL/kg). The total dose is normally injected within 30 to 90 seconds. A higher dosage may be indicated where poor visualization is anticipated (e.g., elderly patients, obese patients, patients with impaired renal function or patients in whom dense opacification of the pelvo-calyceal system and ureters is desired). In these patients, a dose of 100 to 150 mL (1.5 to 2.0 mL/kg) may be used.
Children — The following schedule is recommended for infants and children.
| Under 6 months of age | 3 mL/kg |
| Over 6 months of age | 2 mL/kg |
| The total dosage in children should not exceed | 5 mL/kg |
CONTRAST ENHANCEMENT OF COMPUTED TOMOGRAPHIC (CT) HEAD IMAGING
HEXABRIX may be useful to enhance the presence and better define the extent of primary and metastatic malignancies of the head. In cases where lesions have calcified, there is less likelihood of enhancement. Following therapy, tumors may show decreased or no enhancement.
The use of HEXABRIX may also be beneficial in the image enhancement of non-neoplastic lesions, such as cerebral infarcts, sites of active infection, arterio-venous malformations and aneurysms.
The opacification of the inferior vermis occurs occasionally in normal studies.
CONTRAST ENHANCEMENT IN BODY COMPUTED TOMOGRAPHY
Patient Preparation
No special patient preparation is required. However, it is advisable to insure that patients are well hydrated. In patients undergoing abdominal or pelvic examination, opacification of the bowel may be valuable in scan interpretation.
Precautions
In addition to the general precautions described, patient cooperation is essential since patient motion, including respiration, can markedly affect image quality. The use of an intravascular contrast medium can obscure tumors in patients undergoing CT evaluation of the liver resulting in a false negative diagnosis. Dynamic CT scanning is the procedure of choice for malignant tumor enhancement. (See CLINICAL PHARMACOLOGY.)
Usual Dosage
HEXABRIX may be administered by bolus injection, rapid infusion or by a combination of both. Depending on the area to be examined, doses of 30-150 mL (0.4-0.9 mL/lb) may be administered. When prolonged enhancement is required up to 150 mL can be used, usually with 25-50 mL as a rapid bolus and the remainder as an infusion.
ARTHROGRAPHY
Due to the low osmolality of HEXABRIX, the concomitant use of epinephrine is not necessary since the rate of contrast medium absorption as well as the production of synovial fluid and consequent dilution of the medium are reduced.
Precautions
In addition to the general precautions previously described, strict aseptic technique is required to prevent the introduction of infection. Fluoroscopic control should be used to insure proper introduction of the needle into the synovial space and prevent extracapsular injection. Aspiration of excessive synovial fluid will reduce the pain on injection and prevent the dilution of the contrast agent. It is important that undue pressure not be exerted during the injection.
Adverse Reactions
In addition to the general adverse reactions previously described, arthrography may induce joint pain or discomfort which is usually mild and transient but occasionally may be severe and persist for 24 to 48 hours following the procedure. Effusion requiring aspiration may occur in patients with rheumatoid arthritis.
Usual Dosage
Arthrography is usually performed under local anesthesia. The amount of contrast agent required is solely dependent on the size of the joint to be injected and the technique employed.
The following dosage schedule for normal adult joints should serve only as a guide since joints may require more or less contrast medium for optimal visualization.
| Knee, hip | 5-15 mL | |
| Shoulder, ankle | 5-20 mL | |
| Temporomandibular | 0.5-0.7 mL |
Passive or active manipulation is used to disperse the medium throughout the joint space.
The lower volumes of contrast medium are usually employed for double contrast examinations in which 30-100 cc of either filtered room air or carbon dioxide may be introduced for examination of the knee and lesser volumes for other joints.
HYSTEROSALPINGOGRAPHY
Patient Preparation
It is preferable to perform the procedure approximately eight to ten days after the onset of menses. The patient should empty the bladder before the examination.
Precautions
Caution should be exercised in patients suspected of having cervical or tubal carcinoma to avoid possible spread of the lesion by the procedure. Delayed onset of pain and fever (1-2 days) may be indicative of pelvic infection.
How is Hexabrix supplied
| HEXABRIX Glass Vials/Bottles | NDC Number |
|---|---|
| 10x20 mL vials | 0019-5505-51 |
| 25x50 mL vials | 0019-5505-06 |
| 12x100 mL fill/150 mL bottles | 0019-5505-08 |
| 12x200 mL fill/250 mL bottles | 0019-5505-21 |
Storage:
Store below 30°C (86°F). Do not freeze. If product is frozen or if crystallization of the salt has occurred, examine the container for physical damage. If no damage has occurred, the container should be brought to room temperature. Shake vigorously to assure complete dissolution of any crystals. The speed of dissolution may be increased by heating with circulating warm air. Before use, examine the product to assure that all solids are dissolved and that the container and closure have not been damaged.
This preparation is sensitive to light and must be protected from strong daylight or direct exposure to the sun.
As with all contrast media, glass containers should be inspected prior to use to ensure that breakage or other damage has not occurred during shipping and handling. All containers should be inspected for closure integrity. Damaged containers should not be used.
Mallinckrodt, the “M” brand mark and the Mallinckrodt Pharmaceuticals logo are trademarks of a Mallinckrodt company. © 2014 Mallinckrodt.
† U.S. Registered Trademark of Guerbet S.A., used under license.
Manufactured by:
Mallinckrodt Inc.
Raleigh, NC 27616
www.Mallinckrodt.com
Made in USA
MKR 55050315
Revised 3/2015
Mallinckrodt Pharmaceuticals
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Label
For Intravascular Use
Sterile Solution
200 mL 5505-21
HEXABRIX
Ioxaglate Meglumine 39.3% and Ioxaglate Sodium 19.6% Injection USP
320 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx Only
†Licensed by Guerbet, S.A. Registered U.S. Patent and Trademark Office.
Protect from light •
Store below 30°C (86°F). Do not freeze.
Each mL contains 393 mg ioxaglate meglumine, 196 mg ioxaglate
sodium and 0.10 mg edetate calcium disodium. The pH is adjusted
with meglumine, sodium hydroxide or ioxaglic acid.
Single dose container • Discard unused portion
Usual Dosage:
See Package Insert.
13010110
Mallinckrodt Inc.
St. Louis, MO 63042 USA
www.Mallinckrodt.com

| HEXABRIX
ioxaglate meglumine and ioxaglate sodium injection |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Mallinckrodt Inc. (047021092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mallinckrodt Inc. | 109024984 | ANALYSIS(0019-5505) , MANUFACTURE(0019-5505) | |
More about Hexabrix (ioxaglate)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: ionic iodinated contrast media
- Breastfeeding
