Pfizer-BioNTech Vaccine: Package Insert / Prescribing Info
Package insert / product label
Generic name: covid-19 vaccine, mrna
Dosage form: injection, suspension
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Oct 17, 2023.
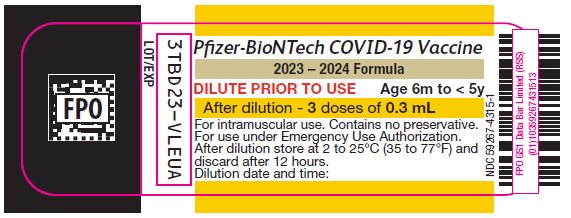
PRINCIPAL DISPLAY PANEL – 3 doses of 0.3 mL Multiple Dose Vial Label
Pfizer-BioNTech COVID-19 Vaccine
2023 – 2024 Formula
DILUTE PRIOR TO USE
Age 6m to < 5y
After dilution – 3 doses of 0.3 mL
For intramuscular use. Contains no preservative.
For use under Emergency Use Authorization.
After dilution store at 2 to 25°C (35 to 77°F) and
discard after 12 hours.
Dilution date and time:
NDC 59267-4315-1
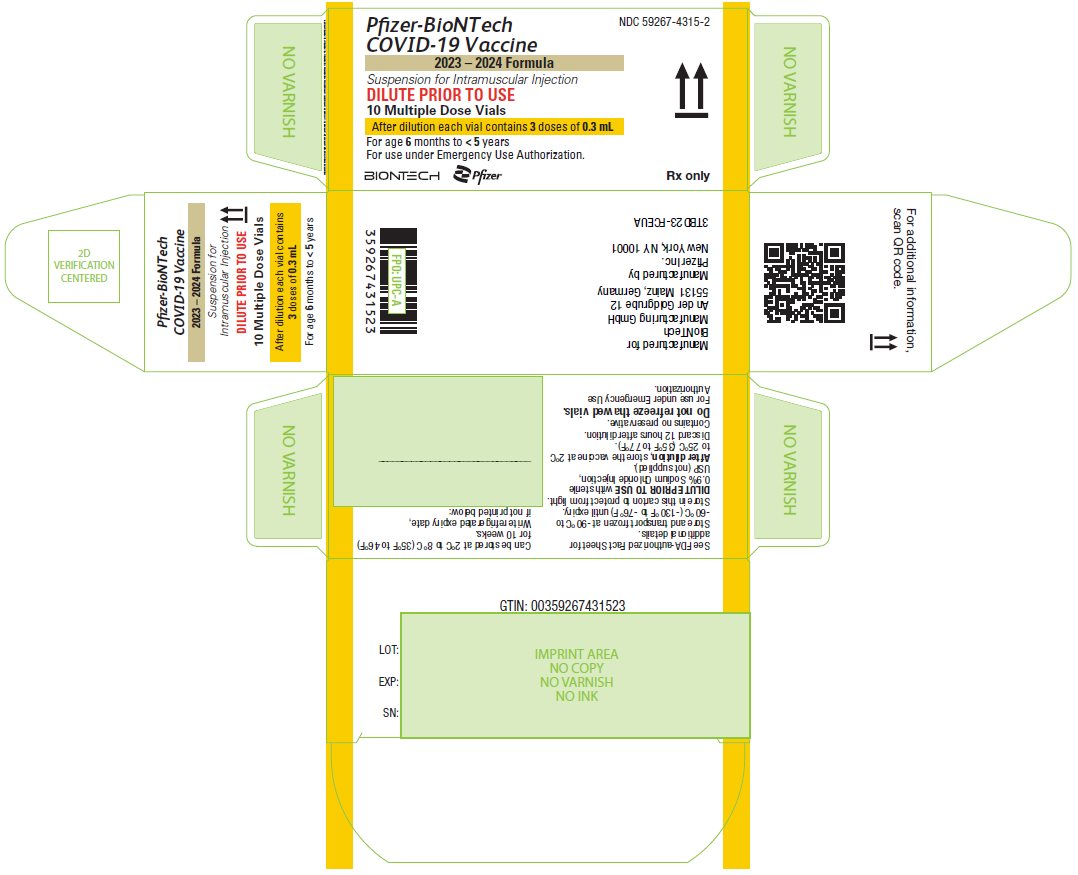
PRINCIPAL DISPLAY PANEL – 10 Multiple Dose Vial Carton
NDC 59267-4315-2
Pfizer-BioNTech
COVID-19 Vaccine
2023 – 2024 Formula
Suspension for Intramuscular Injection
DILUTE PRIOR TO USE
10 Multiple Dose Vials
After dilution each vial contains 3 doses of 0.3 mL
For age 6 months to < 5 years
For use under Emergency Use Authorization.
BIONTECH
Pfizer
Rx only
Related/similar drugs
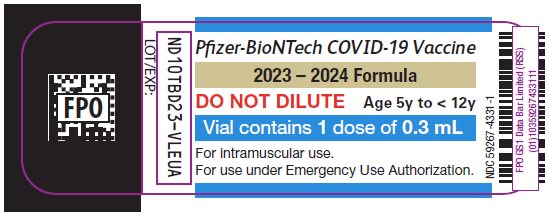
PRINCIPAL DISPLAY PANEL – 0.3 mL Single Dose Vial Label
Pfizer-BioNTech COVID-19 Vaccine
2023 – 2024 Formula
DO NOT DILUTE
Age 5y to < 12y
Vial contains 1 dose of 0.3 mL
For intramuscular use.
For use under Emergency Use Authorization.
NDC 59267-4331-1
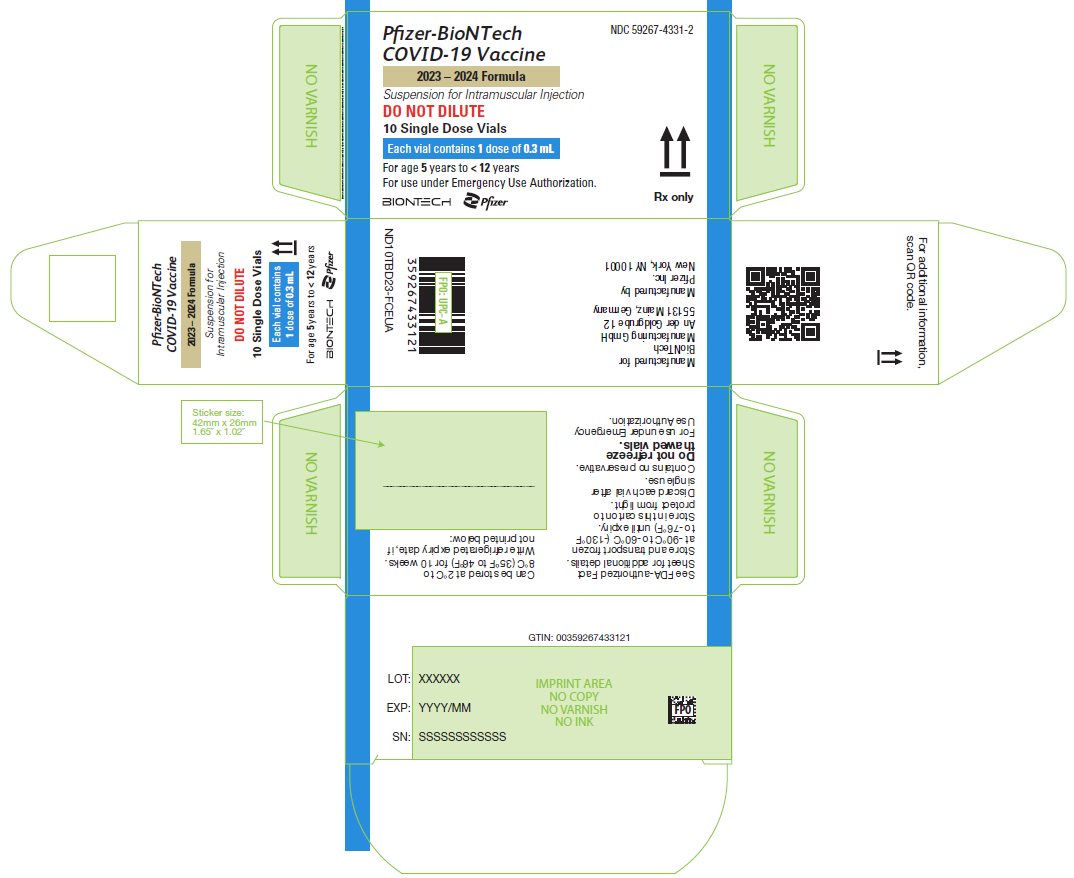
PRINCIPAL DISPLAY PANEL – 10 Single Dose Vial Carton
NDC 59267-4331-2
Pfizer-BioNTech
COVID-19 Vaccine
2023 – 2024 Formula
Suspension for Intramuscular Injection
DO NOT DILUTE
10 Single Dose Vials
Each vial contains 1 dose of 0.3 mL
For age 5 years to < 12 years
For use under Emergency Use Authorization.
BIONTECH
Pfizer
Rx only
| PFIZER-BIONTECH COVID-19 VACCINE
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PFIZER-BIONTECH COVID-19 VACCINE
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Manufacturing Belgium NV (370156507) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | PACK(59267-4315, 59267-4331) , MANUFACTURE(59267-4315, 59267-4331) , ANALYSIS(59267-4315, 59267-4331) , LABEL(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC | 174350868 | ANALYSIS(59267-4315, 59267-4331) , API MANUFACTURE(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing GmbH | 314382536 | ANALYSIS(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing Marburg GmbH | 313270335 | ANALYSIS(59267-4315, 59267-4331) , API MANUFACTURE(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985586408 | ANALYSIS(59267-4315, 59267-4331) , API MANUFACTURE(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Labor LS SE & Co. KG | 314929072 | ANALYSIS(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Innovative Manufacturing Services GmbH | 537365801 | ANALYSIS(59267-4315, 59267-4331) | |
Frequently asked questions
More about COVID-19 mRNA (Pfizer) vaccine
- Check interactions
- Compare alternatives
- Reviews (17)
- Latest FDA alerts (1)
- Side effects
- Dosage information
- During pregnancy
- Drug class: viral vaccines
Patient resources
Professional resources
- COVID-19 Vaccine, mRNA (Pfizer-BioNTech) (2024-2025 Formula) monograph
- Covid-19 Vaccine Pfizer (FDA)
- Covid-19 Vaccine Pfizer, Bivalent (FDA)
- Covid-19 Vaccine Pfizer, Monovalent, 6 mo-4 yr (FDA)
- Covid-19 Vaccine Pfizer, Monovalent,5 yr to 11 yr (FDA)