Penpulimab kcqx: Package Insert / Prescribing Info
Package insert / product label
Dosage form: injection
Drug class: Anti-PD-1 and PD-L1 monoclonal antibodies (immune checkpoint inhibitors)
Medically reviewed by Drugs.com. Last updated on May 2, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Medication Guide
Highlights of Prescribing Information
PENPULIMAB-KCQX injection, for intravenous use
Initial U.S. Approval: 2025
Indications and Usage for Penpulimab kcqx
Penpulimab-kcqx is a programmed death receptor-1 (PD-1)-blocking antibody indicated:
in combination with either cisplatin or carboplatin and gemcitabine for the first-line treatment of adults with recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (NPC) (
1.1)
as a single agent for the treatment of adults with metastatic non-keratinizing NPC with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy. (
1.2)
Penpulimab kcqx Dosage and Administration
Penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine:
- 200 mg intravenously over 60 minutes every 3 weeks until disease progression or a maximum of 24 months. ( 2.1)
Penpulimab-kcqx as a single agent:
- 200 mg intravenously over 60 minutes every 2 weeks until disease progression or a maximum of 24 months. ( 2.2)
Dilute prior to administration. ( 2.4)
Dosage Forms and Strengths
Injection: 100 mg/10 mL (10 mg/mL) solution in a single-dose vial.(3) (3)
Contraindications
None.(4) (4)
Warnings and Precautions
- Immune-Mediated Adverse Reactions ( 5.1)
o Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis and hepatotoxicity, immune-mediated
endocrinopathies, immune-mediated dermatologic adverse reactions, immune-mediated nephritis and renal dysfunction, immune-mediated dermatologic adverse reactions and solid organ transplant rejection.
o Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment.
o Withhold or permanently discontinue penpulimab-kcqx based on severity and type of reaction.
- Infusion-Related Reactions: Interrupt, slow the rate of infusion, or permanently discontinue penpulimab-kcqx based on the severity of the reaction. ( 5.2)
- Complications of Allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. ( 5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. ( 5.4, 8.1, 8.3)
Adverse Reactions/Side Effects
Penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine: The most common adverse reactions (≥20%) were nausea, vomiting, hypothyroidism, constipation, decreased appetite, decreased weight, cough, COVID-19 infection, fatigue, rash, and pyrexia. (6.1) (6)
Penpulimab-kcqx as a single agent: The most common adverse reactions (≥20%) were anemia and hypothyroidism. (6.1) (6)
(6)
To report SUSPECTED ADVERSE REACTIONS, contact Akeso Biopharma Co., Ltd. at toll-free phone 833-662-5376 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
Use In Specific Populations
Lactation: Advise not to breastfeed ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
See 17 for Medication Guide.
Revised: 4/2025
Full Prescribing Information
1. Indications and Usage for Penpulimab kcqx
1.1 First-line Treatment of Recurrent or Metastatic Non-Keratinizing Nasopharyngeal Carcinoma
Penpulimab-kcqx, in combination with either cisplatin or carboplatin and gemcitabine, is indicated for the first-line treatment of adults with recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (NPC).
2. Penpulimab kcqx Dosage and Administration
2.1 Recommended Dosage of Penpulimab-kcqx in Combination with Either Cisplatin or Carboplatin and Gemcitabine – Every 3 Week Dosing
First-line Treatment of Recurrent or Metastatic Nasopharyngeal Carcinoma
The recommended dosage of penpulimab-kcqx is 200 mg administered as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity, for a maximum of 24 months.
Table 1 Order of Administration and Regimen for Penpulimab-kcqx in Combination with Either Cisplatin or Carboplatin and Gemcitabine
|
Administer the regimen in the following order: penpulimab-kcqx first, gemcitabine second and cisplatin or carboplatin last. |
|
|
Drug and Dose |
Duration/ Timing of Treatment |
|
penpulimab-kcqx 200 mg intravenously |
Every 3 weeks for 6 cycles, and then every 3 weeks until unacceptable toxicity or disease progression for a maximum of 24 months |
|
gemcitabine 1000 mg/m 2 intravenously |
Every 3 weeks for 6 cycles |
|
cisplatin 80 mg/m 2 intravenously or carboplatin AUC 5 intravenously |
Every 3 weeks for 6 cycles |
2.2 Recommended Dosage of Penpulimab-kcqx as a Single Agent – Every 2 Week Dosing
Recurrent Metastatic Non-Keratinizing Nasopharyngeal Carcinoma
The recommended dosage of penpulimab-kcqx is 200 mg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity, for a maximum of 24 months.
2.3 Dosage Modifications of Penpulimab-kcqx for Adverse Reactions
No dose reduction for penpulimab-kcqx is recommended. In general, withhold penpulimab-kcqx for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue penpulimab-kcqx for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating steroids.
Dosage modifications for penpulimab-kcqx in combination or penpulimab-kcqx as a single agent for adverse reactions that require management different from these general guidelines are summarized in Table 2.
Table2Recommended Dosage Modification forAdverse Reactions
|
Adverse Reaction |
Severity* |
Dosage Modification |
|
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] |
||
|
Pneumonitis |
Grade 2 |
Withhold † |
|
Grade 3 or 4 |
Permanently discontinue |
|
|
Colitis |
Grade 2 or 3 |
Withhold † |
|
Grade 4 |
Permanently discontinue |
|
|
Hepatitis with no tumor involvement of the liver |
AST or ALT increases to more than 3 and up to 8 times ULN or Total bilirubin increases to more than 1.5 and up to 3 times ULN |
Withhold † |
|
AST or ALT increases to more than 8 times ULN or Total bilirubin increases to more than 3 times ULN |
Permanently discontinue |
|
|
Hepatitis with tumor involvement of the liver ‡ |
Baseline AST or ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN or Baseline AST or ALT is more than 3 and up to 5 times ULN and increases to more than 8 and up to 10 times ULN |
Withhold † |
|
ALT or AST increases to more than 10 times ULN or Total bilirubin increases to more than 3 times ULN |
Permanently discontinue |
|
|
Endocrinopathies |
Grade 3 or 4 |
Withhold until clinically stable or permanently discontinue depending on severity |
|
Nephritis with Renal Dysfunction |
Grade 2 or 3 increased blood creatinine |
Withhold † |
|
Grade 4 increased blood creatinine |
Permanently discontinue |
|
|
Exfoliative Dermatologic Conditions |
Suspected SJS, TEN, or DRESS |
Withhold † |
|
Confirmed SJS, TEN, or DRESS |
Permanently discontinue |
|
|
Myocarditis |
Grade 2, 3, or 4 |
Permanently discontinue |
|
Neurological Toxicities |
Grade 2 |
Withhold † |
|
Grade 3 or 4 |
Permanently discontinue |
|
|
Other Adverse Reactions |
||
|
Infusion-Related Reactions [seeWarnings and Precautions (5.2) ] |
Grade 1 or 2 |
Interrupt or slow the rate of infusion |
|
Grade 3 or 4 |
Permanently discontinue |
|
*Based on Common Terminology Criteria for Adverse Events (CTCAE), version 5.0
†Resume in patients with complete or partial resolution (Grades 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating steroids or inability to reduce prednisone to 10 mg per day or less (or equivalent) within 12 weeks of initiating steroids.
‡If AST and ALT are less than or equal to ULN at baseline, withhold or permanently discontinue penpulimab-kcqx based on recommendations for hepatitis with no liver involvement.
ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson Syndrome, TEN = toxic epidermal necrolysis, ULN = upper limit of normal
2.4 Preparation and Administration
Preparation and Storage of Diluted Solution
- Inspect the solution for particulate matter and discoloration. The solution is clear to slightly opalescent, colorless to yellowish. Discard the vial if the solution is cloudy, discolored, or contains visible particles.
- Dilute penpulimab-kcqx injection prior to intravenous administration.
- Withdraw the required volume from two vials of penpulimab-kcqx and transfer into an intravenous (IV) bag containing 100 mL or less of 0.9% Sodium Chloride Injection, USP.
- Gently invert the bag to mix the diluted solution .Do not shake.
- The final concentration of the diluted solution should be between 2 mg/mL to 5 mg/mL.
- Discard any unused portion left in the vial.
- The product does not contain a preservative.
- Administer diluted solution immediately once prepared. If diluted solution is not administered immediately the total time from dilution to the end of the infusion should not exceed 4 hours. Store the diluted solution up to 4 hours either at room temperature 20°C to 25°C (68°F to 77°F), or refrigerated at 2°C to 8°C (36°F to 46°F). Discard after 4 hours.
- Do not freeze.
Administration
- Administer diluted solution intravenously over 60 minutes through an intravenous line containing a sterile, non-pyrogenic, low-protein binding 0.2 micron to 0.22 micron in-line or add-on filter.
- Do not co-administer other drugs through the same infusion line.
3. Dosage Forms and Strengths
Injection: 100 mg/10 mL (10 mg/mL) as a clear to slightly opalescent, colorless to yellowish solution in a single-dose vial.
5. Warnings and Precautions
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
Penpulimab-kcqx is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death-receptor 1 (PD-1) or the PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under WARNINGS AND PRECAUTIONS may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue and can affect more than one body system simultaneously. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD- L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue penpulimab-kcqx depending on severity [see Dosage and Administration (2) ].In general, if penpulimab-kcqx requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month [ see Dosage and A dministration (2)]. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
Penpulimab-kcqx can cause immune-mediated pneumonitis. In patients treated with other PD-1/PD-L1 blocking antibodies, the incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
Penpulimab-kcqx in Combinationwith EitherCisplatin or Carboplatin and Gemcitabine
Immune-mediated pneumonitis occurred in 0.7% (1/146) of patients receiving penpulimab-kcqx, which was a Grade 2 (0.7%) adverse reaction. Systemic corticosteroids were required in the patient. Pneumonitis led to withholding of penpulimab-kcqx in the patient.
Penpulimab-kcqx as a Single Agent
Immune-mediated pneumonitis occurred in 1.3% (5/372) of patients receiving penpulimab-kcqx, including Grade 3 (0.5%), Grade 2 (0.5%) and Grade 1 (0.3%) adverse reactions. Systemic corticosteroids were required in 80% (4/5) of patients with pneumonitis. Pneumonitis led to permanent discontinuation of penpulimab-kcqx in 0.8% (3/372). Pneumonitis resolved in 20% (1/5) of these patients.
Immune-Mediated Colitis
Penpulimab-kcqx can cause immune-mediated colitis, which may present with diarrhea. Cytomegalovirus (CMV) infection/reactivation can occur in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
Penpulimab-kcqx as a Single Agent
Immune-mediated colitis occurred in 1.1% (4/372) of patients receiving penpulimab-kcqx, including Grade 2 (0.8%) and Grade 1 (0.3%) adverse reactions. Systemic corticosteroids were required in 75% (3/4) of patients with colitis. Colitis led to withholding of penpulimab-kcqx in 0.8% (3/372) of patients.
Immune-Mediated Hepatitis and Hepatotoxicity
Penpulimab-kcqx can cause immune-mediated hepatitis.
Penpulimab-kcqx in Combinationwith Either Cisplatin or Carboplatin and Gemcitabine
Immune-mediated hepatitis occurred in 0.7% (1/146) of patients receiving penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, which was a Grade 2 (0.7%) adverse reaction. Systemic corticosteroids and withholding of penpulimab-kcqx were required in this patient.
Penpulimab-kcqx as a Single Agent
Immune-mediated hepatitis occurred in 3.8% (14/372) of patients receiving penpulimab-kcqx, including Grade 3 (0.8%), Grade 2 (1.3%) and Grade 1 (1.6%) adverse reactions. Systemic corticosteroids were required in 14% (2/14) of patients with hepatitis. Hepatitis led to permanent discontinuation of penpulimab-kcqx in 0.3% (1/372) and withholding of penpulimab-kcqx in 2.2% (8/372) of patients. Hepatitis resolved in 64% (9/14) of these patients.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
Penpulimab-kcqx can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold penpulimab-kcqx depending on severity [see Dosage and Administration (2.2)].
Hypophysitis
Penpulimab-kcqx can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement as indicated. Withhold or permanently discontinue penpulimab-kcqx depending on severity [see Dosage and Administration (2.2)].
Thyroid Disorders:
Penpulimab-kcqx can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated. Withhold or permanently discontinue penpulimab-kcqx based on the severity [seeDosage and Administration (2) ] .
Penpulimab-kcqx in Combinationwith Either Cisplatin or Carboplatin and Gemcitabine
Hyperthyroidism:Immune-mediated hyperthyroidism occurred in 2.1% (3/146) of patients receiving penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, including Grade 2 (0.7%) and Grade 1 (1.4%) adverse reactions.
Hypothyroidism:Immune-mediated hypothyroidism occurred in 16% (26/146) of patients receiving penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, including Grade 2 (13%) and Grade 1 (4.8%) adverse reactions. Penpulimab-kcqx was withheld in 0.7% (1/146) of patients. Hypothyroidism resolved in 12% (3/26) of these patients.
Penpulimab-kcqx as a Single Agent
Thyroiditis:Thyroiditis occurred in 0.5% (2/372) of patients receiving penpulimab-kcqx, including Grade 2 (0.3%) and Grade 1 (0.3%) adverse reactions. Thyroiditis led to withholding of penpulimab-kcqx in 0.3% (1/372) of patients. Thyroiditis was resolved in 50% (1/2) of patients.
Hyperthyroidism:Immune-mediated hyperthyroidism occurred in 7% (24/372) of patients receiving penpulimab-kcqx, including Grade 2 (1.1%) and Grade 1 (5%) adverse reactions. Penpulimab-kcqx was withheld in 0.5% (2/372) of patients. Hyperthyroidism resolved in 79% (19/24) of these patients.
Hypothyroidism:Immune-mediated hypothyroidism occurred in 19% (69/372) of patients receiving penpulimab-kcqx, including Grade 2 (7%) and Grade 1 (12%) adverse reactions. Penpulimab-kcqx was withheld in 1.6% (6/372) of patients. Hypothyroidism resolved in 48% (33/69) of these patients.
Type 1 Diabetes Mellitus, which can present with Diabetic Ketoacidosis
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold penpulimab-kcqx depending on severity [seeDosage and Administration (2) ].
Penpulimab-kcqx in Combinationwith Either Cisplatin or Carboplatin and Gemcitabine
Diabetes occurred in 2.7% (4/146) of patients receiving penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, including Grade 4 (0.7%), Grade 3 (0.7%), Grade 2 (0.7%) and Grade 1 (0.7%) adverse reactions. Diabetes led to permanent discontinuation of penpulimab-kcqx in 0.7% (1/146) and withholding of penpulimab-kcqx in 0.7% (1/146) of patients. Diabetes was resolving in 75% (3/4) of these patients.
Penpulimab-kcqx as a Single Agent
Diabetes occurred in 0.8% (3/372) of patients receiving penpulimab-kcqx as a single agent, including Grade 1 (0.8%) adverse reactions. Diabetes resolved in 67% (2/3) of these patients.
Immune-Mediated Nephritis with Renal Dysfunction
Penpulimab-kcqx can cause immune-mediated nephritis and renal dysfunction.
Penpulimab-kcqx as a Single Agent
Immune-mediated nephritis occurred in 0.5% (2/372) of patients receiving penpulimab-kcqx, including Grade 3 (0.3%) and Grade 2 (0.3%) adverse reactions. Immune-mediated nephritis led to permanent discontinuation of penpulimab-kcqx in 0.5% (2/372) of patients. Systemic corticosteroids were required in 50% (1/2) of patients. Nephritis resolved in 50% (1/2) of these patients.
Immune-Mediated DermatologicAdverseReactions
Penpulimab-kcqx can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens Johnson Syndrome (SJS), Drug Rash with Eosinophilia and Systemic Symptoms (DRESS), and Toxic Epidermal Necrolysis (TEN) has occurred with PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue penpulimab-kcqx depending on severity [seeDosage and Administration (2)].
Penpulimab-kcqx in Combinationwith Either Cisplatin or Carboplatin and Gemcitabine
Immune-mediated rash or dermatitis occurred in 10% (14/146) of patients receiving penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, including Grade 3 (0.7%), Grade 2 (4.8%) and Grade 1 (4.1%) adverse reactions. Systemic corticosteroids were required in 36% (5/14) of patients with immune-mediated rash or dermatitis. Immune-mediated rash or dermatitis led to withholding of penpulimab-kcqx in 1.4% (2/146) of patients. Immune-mediated rash or dermatitis resolved in 78.6% (11/14) of these patients.
Penpulimab-kcqx as a Single Agent
Immune-mediated rash or dermatitis occurred in 12% (43/372) of patients receiving penpulimab-kcqx, including Grade 3 (1.3%), Grade 2 (3.5%) and Grade 1 (7%) adverse reactions. Systemic corticosteroids were required in 14% (6/43) of patients with immune-mediated rash or dermatitis. Immune-mediated rash or dermatitis led to permanent discontinuation of penpulimab-kcqx in 0.3% (1/372) and withholding of penpulimab-kcqx in 1.6% (6/372) of patients. Immune-mediated rash or dermatitis resolved in 58% (25/43) of these patients.
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine or single-agent penpulimab-kcqx or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions.
Cardiac/vascular:Myocarditis, pericarditis, vasculitis.
Nervous system:Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy, nerve injury.
Ocular:Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment including blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal:Pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and Connective Tissue:Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine:Hypoparathyroidism.
Hematologic/Immune:Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection, other transplant (including corneal graft) rejection.
5.2 Infusion-Related Reactions
Penpulimab-kcqx can cause severe or life-threatening infusion-related reactions, including hypersensitivity and anaphylaxis. Discontinue penpulimab-kcqx in patients with severe or life-threatening infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild or moderate infusion-related reactions [see Dosage and Administration (2) ].
Penpulimab-kcqx in Combinationwith Either Cisplatin or Carboplatin and Gemcitabine
Infusion-related reactions occurred in 3.4% of 146 patients receiving penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, including Grade 2 (1.4%) and Grade 1 (2.1%) infusion related reactions.
Penpulimab-kcqx as a Single Agent
Infusion-related reactions occurred in 10% of 372 patients receiving penpulimab-kcqx as single agent, including Grade 4 (0.3%), Grade 3 (0.3%), Grade 2 (6%) and Grade 1 (3.8%) infusion related reactions. Penpulimab-kcqx was withheld in 0.8% (3/372) and permanently discontinued in 0.3% (1/372) of patients.
5.3 Complications of Allogeneic HSCT
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause) [seeAdverse Reactions (6.1)].These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and Allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an Allogeneic HSCT.
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, penpulimab-kcqx can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death. Advise pregnant women of the potential risk to a fetus.
Advise females of reproductive potential to use effective contraception during treatment with penpulimab-kcqx and for 4 months after the last dose [see Use in Specific Populations ( 8.1 , 8.3)].
6. Adverse Reactions/Side Effects
The following adverse reactions are described in other sections of the labeling:
- Severe and Fatal Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1) ]
- Infusion-Related Reactions[see Warnings and Precautions (5.2) ]
- Complications of Allogeneic HSCT [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to those observed in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS reflect exposure to penpulimab-kcqx in 146 patients in Study AK105-304 [see Clinical Studies (14.1) ]. Patients received 6 cycles every 3 weeks of intravenous penpulimab-kcqx 200 mg in combination with either cisplatin 80 mg/m 2 or carboplatin AUC 5 and gemcitabine 1000 mg/m 2 followed by single-agent penpulimab-kcqx 200 mg every 3 weeks until disease progression or a maximum of 24 months. Among the 146 patients who received penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, 71% were exposed for 6 months or longer and 38% were exposed for greater than one year. In this safety population, the most common (≥ 20%) adverse reactions were nausea (58%), vomiting (55%), hypothyroidism (45%), constipation (41%), decreased appetite (36%), decreased weight (26%), cough (25%), COVID-19 infection (25%), fatigue (25%), rash (24%) and pyrexia (21%). The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased lymphocytes (70%), decreased neutrophils (61%), decreased white blood cells (58%), decreased hemoglobin (49%), decreased platelets (33%), decreased potassium (14%), increased lipase (11%), increased ALT (8%), decreased sodium (7%), increased AST (6%), increased triglycerides (4.3%), decreased magnesium (4.2%), decreased CPK (4.1%), increased amylase (2.9%), increased potassium (2.8%), increased cholesterol (2.2%), increased calcium (2.1%) and increased blood bilirubin (2.1%).
The data described in the WARNINGS AND PRECAUTIONS also reflect exposure to single-agent intravenous penpulimab-kcqx 200 mg every 2 weeks until disease progression or a maximum of 24 months in 372 patients in studies: AK105-101 [NCT03352531], AK-105-201 [NCT03722147], AK105-202 [see Clinical Studies (14.2)], AK105-204 [NCT 04172506]. Among the 372 patients who received single-agent penpulimab-kcqx, 49% were exposed for 6 months or longer and 34% were exposed for at least one year. In this safety population, the most common (≥20%) adverse reactions were anemia (25%) and hypothyroidism (23%). The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased lymphocytes (11%), increased GGT (9%), decreased phosphate (6%), decreased sodium (4.7%), increased aspartate aminotransferase (4.4%), increased alkaline phosphatase (4%), decreased hemoglobin (3.6%), increased bilirubin (2.7%), increased glucose (3%), increased triglycerides (2.8%), increased alanine aminotransferase (2.5%), increased magnesium (3.3%), and decreased platelets (2.5%).
First line Recurrent or Metastatic Nasopharyngeal Carcinoma
Study AK105-304
The safety of penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine was evaluated in Study AK105-304 [seeClinical Studies (14.1)]. Patients received intravenous placebo or penpulimab-kcqx 200 mg every 3 weeks in combination with 6 cycles of either cisplatin 80 mg/m 2or carboplatin AUC 5 and gemcitabine 1000 mg/m 2every 3 weeks followed by placebo or penpulimab-kcqx 200 mg intravenously every 3 weeks until unacceptable toxicity, disease progression or a maximum of 24 months. Among patients who received penpulimab-kcqx, 71% were exposed for 6 months or longer and 38% were exposed for greater than one year.
The median age of patients who received penpulimab-kcqx was 51 years (23 to 75 years); 82% were male; 97% were Asian, 3.4% were White; 2.1% were Hispanic or Latino, baseline Eastern Cooperative Oncology Group (ECOG) performance score was 0 (36%) or 1 (64%). Of the 146 patients, 69% of patients had received at least one prior systemic therapy and 100% of patients had received prior radiation therapy.
Serious adverse reactions occurred in 51% of patients. The most frequent serious adverse reactions (≥2%) were thrombocytopenia (19%), decreased neutrophils (14%), decreased white blood cells (12%), anemia (8%), myelosuppression (3.4%), decreased sodium (2.7%), pneumonia (2.1%), and nausea (2.1%). Of the patients who received penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, there was one fatal adverse reaction (0.7%) due to syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Permanent discontinuation of penpulimab-kcqx due to an adverse reaction occurred in 3.4% of patients. Adverse reactions which resulted in permanent discontinuation of penpulimab-kcqx were acute myocardial infarction, acute kidney injury, brain edema, diabetic ketoacidosis, increased potassium, increased lipase, and thrombocytopenia (0.7% each).
Dose interruptions of penpulimab-kcqx due to an adverse reaction occurred in 64% of patients. Adverse reactions which required dose interruption in ≥ 2% of patients included COVID-19 (16%), anemia (16%), thrombocytopenia (14%), decreased white blood cells (14%), decreased neutrophils (10%), pneumonia (10%), increased alanine aminotransferase (3.4%), increased aspartate aminotransferase (2.7%), decreased sodium (2.7%), increased blood creatinine (2.7%), pyrexia (2.1%), and upper respiratory tract infection (2.1%).
Table 3 summarizes the adverse reactions in Study AK105-304.
Table3Adverse Reactions (≥ 10%) in Patients with Recurrent or Metastatic NPC Who Received Penpulimab-kcqx in Combination with Either Cisplatin or Carboplatin and Gemcitabine in Study AK105-304
|
Adverse Reaction |
Penpulimab-kcqx
|
Placebo
|
||
|
All Grades
1
|
Grade 3 or 4
1
|
All Grades
1
|
Grade 3 or 4
1
|
|
|
Gastrointestinal disorders |
||||
|
Nausea |
58 |
2.1 |
64 |
0 |
|
Vomiting 2 |
55 |
1.4 |
54 |
1.4 |
|
Constipation |
41 |
0 |
44 |
0 |
|
Abdominal distension |
12 |
0 |
6 |
0 |
|
Endocrine disorders |
||||
|
Hypothyroidism 3 |
45 |
0 |
27 |
0 |
|
Metabolism and nutrition disorders |
||||
|
Decreased appetite |
36 |
0 |
39 |
0 |
|
Investigations |
||||
|
Weight decreased |
26 |
1.4 |
16 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||||
|
Cough 4 |
25 |
0 |
16 |
0 |
|
Infections and infestations |
||||
|
COVID-19 |
25 |
0 |
17 |
0.7 |
|
General disorders and administration site conditions |
||||
|
Fatigue 5 |
25 |
1.4 |
22 |
0 |
|
Pyrexia |
21 |
0 |
20 |
0.7 |
|
Malaise |
10 |
0 |
8 |
0 |
|
Skin and subcutaneous tissue disorders |
||||
|
Rash 6 |
24 |
1.4 |
20 |
0 |
|
Pruritus |
11 |
0 |
11 |
0 |
|
Nervous system disorders |
||||
|
Headache |
15 |
0.7 |
10 |
0.7 |
|
Dizziness 7 |
14 |
0 |
15 |
0.7 |
|
Renal and urinary disorders |
||||
|
Proteinuria |
14 |
0 |
13 |
0 |
|
Acute kidney injury 8 |
13 |
1.4 |
4.9 |
0 |
|
Musculoskeletal and connective tissue disorders |
||||
|
Musculoskeletal pain 9 |
14 |
0.7 |
24 |
0 |
|
Psychiatric disorders |
||||
|
Insomnia |
12 |
0 |
10 |
0 |
1Graded per NCI CTCAE v5.0
2Includes retching
3Includes blood thyroid stimulating hormone increased, secondary hypothyroidism
4Includes productive cough, upper-airway cough syndrome
5Includes asthenia
6Includes dermatitis, dermatitis acneiform, drug eruption, eczema, rash maculo-papular, rash papular, rash pustular
7Includes vertigo
8Includes acute kidney injury, creatinine renal clearance decreased, glomerular filtration rate decreased, renal failure, renal impairment
9Includes arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, myalgia, neck pain, pain in extremity, spinal pain
Table 4 summarizes the laboratory abnormalities in AK105-304.
Table 4 Select Laboratory Abnormalities (≥20%) That Worsened from Baseline in Patients with Recurrent or Metastatic NPC Who Received Penpulimab-kcqx in Combination with Either Cisplatin or Carboplatin and Gemcitabine in Study AK105-304
| Laboratory Abnormality | Penpulimab-kcqx
Cisplatin or Carboplatin /Gemcitabine 2 | Placebo
Cisplatin or Carboplatin /Gemcitabine 3 |
||
| All Grades
1
(%) | Grade 3 or 4
1
(%) | All Grades
1
(%) | Grade 3 or 4
1
(%) |
|
| Hematology | ||||
| White blood cell decreased | 99 | 58 | 97 | 58 |
| Hemoglobin decreased | 98 | 49 | 97 | 47 |
|
Lymphocytes decreased | 93 | 70 | 89 | 58 |
| Neutrophils decreased | 92 | 61 | 95 | 65 |
| Chemistry | ||||
| Magnesium decreased | 69 | 4.2 | 58 | 3.6 |
| Sodium decreased | 62 | 7 | 58 | 7 |
| Triglycerides increased | 58 | 4.3 | 53 | 4.3 |
| Aspartate aminotransferase increased | 52 | 6 | 37 | 2.9 |
| Cholesterol increased | 49 | 2.2 | 46 | 0.7 |
| Creatinine increased | 48 | 1.4 | 39 | 0.7 |
| Albumin decreased | 47 | 0 | 44 | 1.4 |
| Potassium decreased | 45 | 14 | 31 | 3.6 |
| Alanine aminotransferase increased | 42 | 8 | 37 | 2.1 |
| GGT increased | 36 | 1.4 | 33 | 0.7 |
| Alkaline phosphatase increased | 32 | 0.7 | 30 | 0 |
| Lipase increased | 28 | 11 | 23 | 3.9 |
| Chloride decreased | 25 | 1.4 | 11 | 0 |
| Serum amylase increased | 23 | 2.9 | 17 | 0.7 |
| Glucose decreased | 22 | 1.4 | 20 | 0.7 |
| Lipids increased | 21 | 1.4 | 20 | 0.7 |
1Toxicity graded according to NCI CTCAE v5.0.
2The denominator used to calculate the rate varied from 102 to 146 based on the number of patients with a baseline value and at least one post-treatment value.
3The denominator used to calculate the rate varied from 103 to 142 based on the number of patients with a baseline value and at least one post-treatment value.
Recurrent Metastatic Non-Keratinizing Nasopharyngeal Carcinoma
Study AK105-202
The safety of penpulimab-kcqx as a single agent was evaluated in Study AK105-202 [seeClinical Studies (14.2)].Eligible patients had metastatic non-keratinizing NPC and had received prior platinum-based chemotherapy and at least one other line of therapy or had disease progression within 6 months of completion of platinum-based chemotherapy administered as neoadjuvant, adjuvant, or definitive chemoradiation treatment. Patients received penpulimab-kcqx 200 mg intravenously every 2 weeks until unacceptable toxicity, disease progression, or a maximum of 24 months. Among patients who received penpulimab-kcqx, 45% were exposed for 6 months or longer and 30% for exposed for greater than one year.
The median age of patients who received penpulimab-kcqx was 50 years (20 to 66 years); 76% were male; 100% were Asian, 100% had recurrent disease; baseline Eastern Cooperative Oncology Group (ECOG) performance score was 0 (31%) or 1 (69%). All patients had received at least one prior systemic therapy and prior radiation.
Serious adverse reactions occurred in 22% of patients. Serious adverse reactions in ≥1% were pneumonia (3.8%), pneumonitis (1.5%), respiratory failure (1.5%), rash (1.5%). Fatal adverse reactions occurred in 1% of patients treated with penpulimab-kcqx, including a case each of pneumonitis, septic shock, colitis, and hepatitis.
Permanent discontinuation of penpulimab-kcqx due to an adverse reaction occurred in 3.8% of patients. Adverse reactions which resulted in permanent discontinuation of penpulimab-kcqx in ≥1% were rash, hepatitis, herpes zoster, spinal cord compression and pleural effusion (0.8% each).
Dose interruptions of penpulimab-kcqx due to an adverse reaction occurred in 25% of patients. Adverse reactions which required dose interruption in ≥ 1% of patients included hepatitis (6.2%), anemia (3.1%), pneumonia (3.1%), hypothyroidism (3.1%), rash (1.5%) and white blood cells decreased (1.5%).
Table 5 summarizes the adverse reactions in AK105-202.
Table5Adverse Reactions (≥ 10%) in Patients with Recurrent Metastatic Non-Keratinizing NPC Who Received Penpulimab-kcqx in Study AK105-202
|
Adverse Reaction |
Penpulimab-kcqx
|
|
|
All
(%) |
Grade 3 or 4 1 (%) |
|
|
Endocrine disorders |
||
|
Hypothyroidism 2 |
39 |
0 |
|
Musculoskeletal and connective tissue disorders |
||
|
Musculoskeletal pain 3 |
25 |
0.8 |
|
Investigations |
||
|
Weight decreased |
19 |
1.5 |
|
General disorders and administration site conditions |
||
|
Pyrexia |
15 |
0 |
|
Infections and infestations |
||
|
Upper respiratory tract infection |
13 |
0.8 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Cough 4 |
11 |
0 |
|
Skin and subcutaneous tissue disorders |
||
|
Rash 5 |
11 |
2.3 |
1Graded per NCI CTCAE v5.0
2Includes blood thyroid stimulating hormone increased
3Includes arthralgia, back pain, bone pain, musculoskeletal chest pain, neck pain, non-cardiac chest pain, pain in extremity
4Includes productive cough
5Includes dermatitis acneiform, eczema, pemphigoid
Table 6 summarizes the laboratory abnormalities in AK105-202.
Table6Select Laboratory Abnormalities (≥20%) Worsening from Baseline in Patients with Recurrent Metastatic Non-Keratinizing NPC Who Received Penpulimab-kcqx in Study AK105-202
|
Laboratory Abnormality |
Penpulimab-kcqx |
|
|
All Grades 1 (%) 2 |
Grades 3 or 4 (%) |
|
|
Chemistry |
||
|
Creatinine increased |
81 |
0 |
|
Phosphate decreased |
47 |
8 |
|
Sodium decreased |
46 |
6 |
|
Albumin decreased |
45 |
0 |
|
Triglycerides increased |
38 |
3.1 |
|
Alkaline phosphatase increased |
35 |
5 |
|
Aspartate aminotransferase increased |
33 |
3.9 |
|
GGT increased |
34 |
8 |
|
Glucose increased |
34 |
0 |
|
Magnesium decreased |
28 |
0.8 |
|
Activated partial thromboplastin time prolonged |
22 |
0 |
|
Alanine aminotransferase increased |
20 |
3.9 |
|
Hematology |
||
|
Lymphocytes decreased |
43 |
16 |
|
Hemoglobin decreased |
36 |
4.7 |
1 Toxicity graded according to NCI CTCAE v5.0.
2 Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: penpulimab-kcqx (range of the patient number: 128 to130).
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, penpulimab-kcqx can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of penpulimab-kcqx in pregnant women. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death ( see Data). Human immunoglobulin G (IgG) is known to cross the placental barrier; therefore, penpulimab-kcqx has the potential to be transmitted from the mother to the developing fetus. Advise women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Animal reproduction studies have not been conducted with penpulimab-kcqx to evaluate its effect on reproduction and fetal development. A central function of the PD-1/PD-L1 pathway is to preserve pregnancy by maintaining maternal immune tolerance to the fetus. In murine models of pregnancy, blockade of PD-L1 signaling has been shown to disrupt tolerance to the fetus and to result in an increase in fetal loss; therefore, potential risks of administering penpulimab-kcqx during pregnancy include increased rates of abortion or stillbirth. As reported in the literature, there were no malformations related to the blockade of PD-1/PD-L1 signaling in the offspring of these animals; however, immune-mediated disorders occurred in PD-1 and PD-L1 knockout mice. Based on its mechanism of action, fetal exposure to penpulimab-kcqx may increase the risk of developing immune-mediated disorders or of altering the normal immune response.
8.2 Lactation
Risk Summary
There is no information regarding the presence of penpulimab-kcqx in human milk or its effects on the breastfed child, or on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to penpulimab-kcqx are unknown. Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment and for 4 months after the last dose of penpulimab-kcqx.
8.3 Females and Males of Reproductive Potential
Penpulimab-kcqx can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating penpulimab-kcqx [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with penpulimab-kcqx and for 4 months after the last dose.
8.4 Pediatric Use
The safety and effectiveness of penpulimab-kcqx have not been established in pediatric patients [see Indications and Usage (1)].
8.5 Geriatric Use
Penpulimab-kcqx in Combination with Either Cisplatin or Carboplatin and Gemcitabine
Of the 146 patients with NPC who received penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, 15 (10%) were 65 years or older and 1 (0.7%) was 75 years or older. Clinical studies did not include a sufficient number of patients aged 65 years or older to determine differences in safety or effectiveness compared to younger patients [see Adverse Reactions (6)].
Penpulimab-kcqx as a Single-Agent
Of the 372 patients with NPC who received penpulimab-kcqx as a single agent, 69 (19%) were 65 years or older and 7% were 75 years and older. No overall differences in safety or effectiveness were observed between patients age <65 and those ≥ 65 years [see Adverse Reactions (6)].
11. Penpulimab kcqx Description
Penpulimab-kcqx is a programmed death receptor-1 (PD 1)-blocking antibody. Penpulimab-kcqx is a humanized monoclonal IgG1 antibody with an approximate molecular weight of 150 kDa. Penpulimab-kcqx is produced in Chinese hamster ovary (CHO) cells.
Penpulimab-kcqx injection is a sterile, preservative-free, clear to slightly opalescent, colorless to yellowish solution for intravenous infusion after dilution. Each vial contains 100 mg of penpulimab-kcqx in 10 mL solution. Each mL of solution contains: penpulimab-kcqx 10 mg, acetic acid (0.09 mg), polysorbate 80 (0.2 mg), sodium acetate (2.52 mg), sorbitol (45 mg), and Water for Injection, USP. The pH of the solution is 5.8.
12. Penpulimab kcqx - Clinical Pharmacology
12.1 Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Penpulimab-kcqx injection is a humanized IgG1 monoclonal antibody that binds to PD-1 and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In murine tumor models, blocking PD-1 activity resulted in decreased tumor growth.
12.2 Pharmacodynamics
Penpulimab-kcqx exposure-response relationships and the time course of pharmacodynamic response are not fully characterized.
12.3 Pharmacokinetics
Penpulimab-kcqx exposure increased dose proportionally over the dose range of 1 to 10 mg/kg (0.35 to 3.5 times the approved recommended dosage in a 70 kg patient) after the first dose. The mean accumulation ratio was 1.5 for maximum concentration (C max) and 2.1 for area under the concentration curve (AUC) following multiple doses of 200 mg every 3 weeks. Steady-state concentrations of penpulimab-kcqx were reached by Week 15, with mean (CV%) steady-state trough and peak concentrations of 30 μg/mL (38%) and 88 μg/mL (21%), respectively.
Distribution
The mean (CV%) volume of distribution was 10 L (44%).
Elimination
The mean (CV%) clearance was 0.2 L/day (33%) and the mean (CV%) terminal half-life was 32 days (44%).
Metabolism
Penpulimab-kcqx is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of penpulimab-kcqx were observed based on body weight (32 to 113 kg), age (18 to 91 years), sex, race (White and Asian), serum albumin (22 to 54.5 g/L), baseline LDH (111 to 4,450 IU/L), baseline C-reactive protein (0 to 328 mg/L), baseline ECOG score (0 and 1), immunogenicity status, tumor type (NPC and other tumors), co-administration (with chemotherapy), mild or moderate renal impairment (creatinine clearance [CLcr] ≥ 30 mL/min), mild (total bilirubin ≤ ULN and AST > ULN or total bilirubin > 1 to 1.5 times ULN and any AST) or moderate hepatic impairment (total bilirubin > 1.5 to 3 times ULN and any AST).
The effect of severe hepatic (total bilirubin > 3 times ULN and any AST) or renal impairment (CLcr < 30 mL/min) on the pharmacokinetics of penpulimab-kcqx is unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of penpulimab-kcqx or of other penpulimab products.
Of the 349 evaluable patients who received penpulimab-kcqx 1, 3, 10 mg/kg every two weeks or 200 mg every two weeks or every three weeks as a single agent, 30% (105/349) of patients tested positive for anti-drug antibodies (ADA) at one or more post-baseline timepoints. Of those who tested positive for ADA, 17% (18/105) had neutralizing antibodies against penpulimab-kcqx. Of the 371 evaluable patients who received penpulimab-kcqx 200 mg every 3 weeks in combination with chemotherapy, 24% (88/371) of patients tested positive for ADA at one or more post-baseline timepoints. Of those who tested positive for ADA, 44% (39/88) had neutralizing antibodies against penpulimab-kcqx. There are insufficient data to assess whether the observed anti-penpulimab-kcqx antibodies affect the safety or effectiveness of penpulimab-kcqx.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the potential of penpulimab-kcqx for carcinogenicity or genotoxicity.
Fertility studies have not been conducted with penpulimab-kcqx. In 6-week and 3-month repeat dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs; however, most animals in these studies were not sexually mature.
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-1/PD-L1 signaling increased the severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti-PD-1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-1 and PD-L1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
14. Clinical Studies
14.1 First-line Treatment of Recurrent or Metastatic Nasopharyngeal Carcinoma
The efficacy of penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine was evaluated in Study AK105-304 (NCT04974398), a randomized, double-blind, multi-center trial. The trial included a total of 291 patients with recurrent or metastatic nasopharyngeal carcinoma (NPC). Eligible patients were required to have recurrent NPC with local-regional recurrence and/or distant metastasis occurring ≥ 6 months after completion of curative intent treatment or to have primary metastatic NPC not suitable for local therapy at the time of diagnosis. Patients with autoimmune disease, other than stable hypothyroidism or Type I diabetes, and patients who required systemic immunosuppression were ineligible.
Patients were randomized (1:1) to receive either:
- Penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine, every 3 weeks, for up to 6 cycles, followed by single-agent penpulimab-kcqx every 3 weeks until disease progression or unacceptable toxicity or a maximum of 24 months.
- Placebo in combination with either cisplatin or carboplatin and gemcitabine, every 3 weeks for up to 6 cycles, followed by single-agent placebo every 3 weeks until disease progression or unacceptable toxicity or a maximum of 24 months.
All study medications were administered intravenously.
Randomization was stratified by stage of disease (primary metastatic vs. recurrent), Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (0 vs. 1), and liver metastasis (present vs. absent).
Patients randomized to placebo were eligible to receive open-label single-agent penpulimab-kcqx (200 mg every 3 weeks) after radiographic disease progression confirmed by blinded independent central review (BICR).
Tumor assessments were performed every 6 weeks for the first 12 months and every 9 weeks thereafter.
The major efficacy outcome measure was BICR-assessed progression-free survival (PFS) according to RECIST v1.1. The key secondary efficacy outcome measure was overall survival (OS).
The study population characteristics were: median age of 51 years (range: 23 to 75), 82% male, 98% Asian, 2.1% White; 1.4% Hispanic or Latino; and 36% ECOG PS of 0. At study entry, 80% of patients had metastatic disease. Histological subtypes of NPC included 96% non-keratinizing, 0.7% keratinizing squamous cell carcinoma, and 3.4% did not have the subtype identified. In total, 98% received cisplatin and 2.4% received carboplatin.
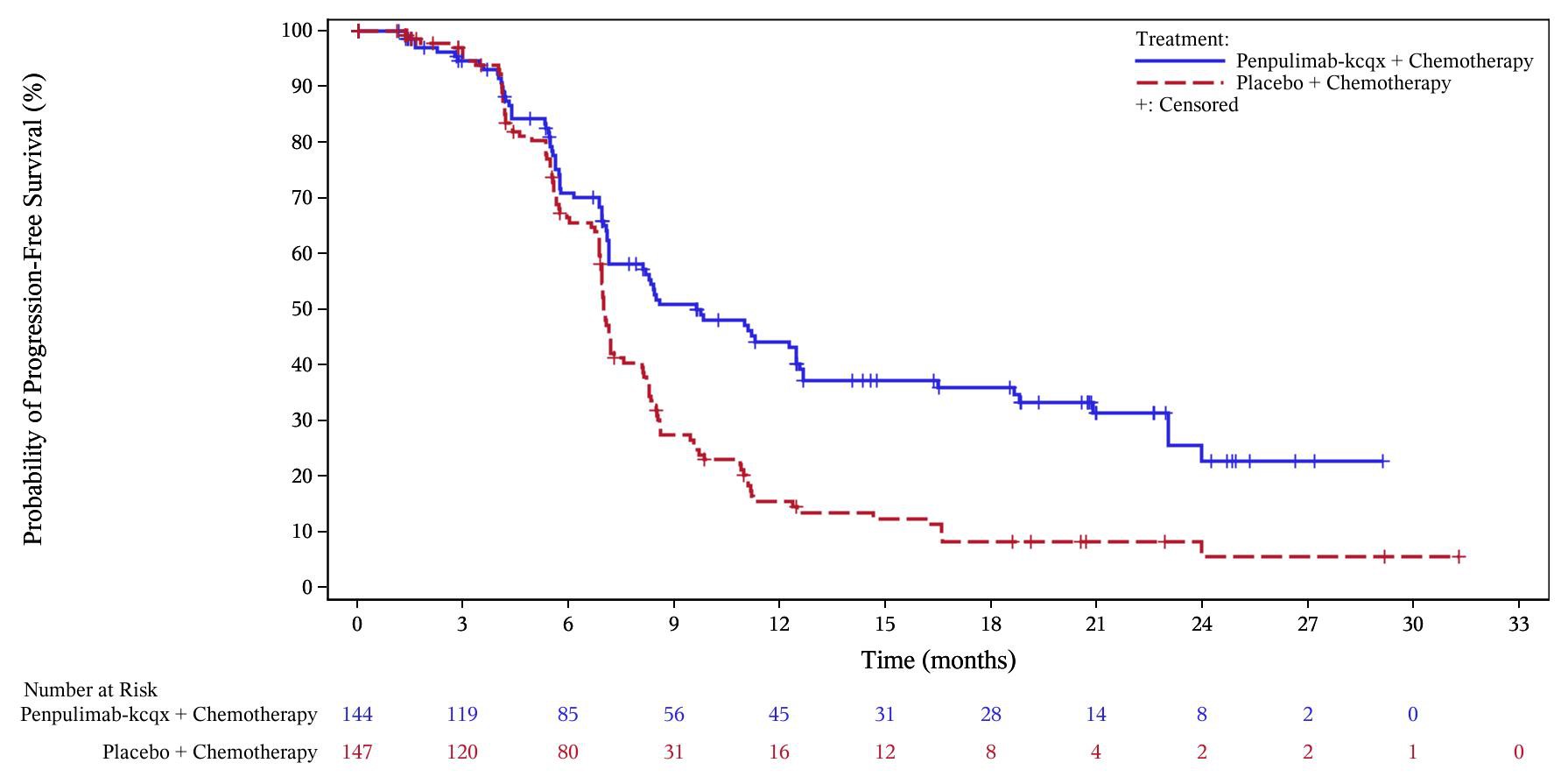
Efficacy results of the analysis of PFS are summarized in Table 7 and Figure 1 below. The trial demonstrated statistically significant improvements in BICR-assessed PFS for patients randomized to penpulimab-kcqx in combination with either cisplatin or carboplatin and gemcitabine compared to placebo with either cisplatin or carboplatin and gemcitabine. OS results were not mature with 70% of pre-specified OS events observed in the overall population.
Table7Efficacy Results in AK105-304
| Endpoint |
Penpulimab-kcqx cisplatin or carboplatin and gemcitabine N=144 |
Placebo cisplatin or carboplatin and gemcitabine N=147 |
|
BICR-assessed Progression-free Survival |
||
| Number of Events n (%) | 80 (56) | 109 (74) |
| Median, months (95% CI) | 9.6 (7.1, 12.5) | 7.0 (6.9, 7.3) |
| Hazard Ratio (95% CI) † | 0.45 (0.33, 0.62) | |
| p-value ‡ | <0.0001 | |
†Based on a stratified Cox proportional hazard model.
‡Two-sided p-value, based on the stratified log-rank test, as compared with an alpha boundary of 0.030.
Figure1Kaplan-Meier Curves of PFS for AK105-304
14.2 Recurrent Metastatic Non-Keratinizing Nasopharyngeal Carcinoma
The efficacy of single-agent penpulimab-kcqx was evaluated in Study AK105-202 (NCT03866967), an open-label, multicenter, single-arm trial conducted in a single country. The trial included a total of 125 patients with unresectable or metastatic non-keratinizing nasopharyngeal carcinoma (NPC) and who had disease progression after platinum-based chemotherapy and at least one other line of therapy. Patients with a history of autoimmune disease or a medical condition that required immunosuppression were ineligible.
Patients received single-agent penpulimab-kcqx 200 mg intravenously every 2 weeks until disease progression or unacceptable toxicity or a maximum of 24 months. Tumor assessments were performed every 8 weeks in the first year and every 12 weeks thereafter. The major efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) according to RECIST v1.1 as assessed by an Independent Radiology Review Committee (IRRC).
The median age was 50 years (range: 21 to 66 years); 76% were male; 100% were Asian; none were Hispanic or Latino; and Eastern Cooperative Oncology Group (ECOG) performance score (PS) was 0 (31%) or 1 (69%). In total, 10% of patients had tumors with PD-L1 TPS <1%, 50% of patients had tumors with PD-L1 TPS 1-49%, 37% of patients had tumors with PD-L1 TPS ≥50%, and 2.4% of patients had tumors with missing PD-L1 expression levels. Sixty-three percent of patients had received 2 prior lines of chemotherapy, and 37% of patients had received 3 or more prior lines of chemotherapy. Ninety-two percent of patients had received prior radiotherapy.
Efficacy results are summarized in Table 8.
Table8Efficacy Results inStudy AK105-202
|
Efficacy Parameter |
Penpulimab-kcqx (N=125) |
|
Objective Response Rate,% (95% CI) |
28 (20, 37) |
|
Complete response rate, % |
0.8 |
|
Partial response rate, % |
27 |
|
Duration of Response (DOR) |
N=35 |
|
Median a, months (95% CI) |
NR (9.2, NE) |
|
Patients with DOR ≥ 12 months (%) b |
46 |
aEstimate using Kaplan-Meier method.
bBased on the observed DOR data.
NR = not reached; NE = not estimable
16. How is Penpulimab kcqx supplied
How Supplied
Penpulimab-kcqx injection is a sterile, preservative-free, clear to slightly opalescent, colorless to yellowish solution supplied as:
- Carton containing one 100 mg/10 mL (10 mg/mL) single-dose vial NDC 83654-105-01
Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
- Do not shake. Do not freeze.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Immune-Mediated Adverse Reactions
Inform patients of the risk of immune-mediated adverse reactions that may be severe or fatal, may occur after discontinuation of treatment, and may require corticosteroid treatment and interruption or discontinuation of penpulimab-kcqx [seeWarnings and Precautions (5.1)]. These reactions may include:
- Pneumonitis: Advise patients to contact their healthcare provider immediately for new or worsening cough, chest pain, or shortness of breath [seeWarnings and Precautions (5.1)].
- Colitis: Advise patients to contact their healthcare provider immediately for diarrhea or severe abdominal pain [seeWarnings and Precautions (5.1)].
- Hepatitis: Advise patients to contact their healthcare provider immediately for jaundice, severe nausea or vomiting, or easy bruising or bleeding [see Warnings and Precautions (5.1) ].
- Endocrinopathies: Advise patients to contact their healthcare provider immediately for signs or symptoms of adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, or Type 1 diabetes mellitus [seeWarnings and Precautions (5.1) ].
- Nephritis: Advise patients to contact their healthcare provider immediately for signs or symptoms of nephritis including decreased urine output, blood in urine, swelling in ankles, loss of appetite, and any other symptoms of renal dysfunction [seeWarnings and Precautions (5.1)].
- Severe skin reactions: Advise patients to contact their healthcare provider immediately for any signs or symptoms of severe skin reactions, Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis, including rash or any other severe skin discomfort [seeWarnings and Precautions (5.1) ].
- Other immune-mediated adverse reactions:
o Advise patients that immune-mediated adverse reactions can occur and may involve any organ system, and to contact their healthcare provider immediately for any new or worsening signs or symptoms [see Warnings and Precautions (5.1) ].
o Advise patients of the risk of solid organ transplant rejection and other transplant (including corneal graft) rejection. Advise patients to contact their healthcare provider immediately for signs or symptoms of organ transplant rejection and other transplant (including corneal graft) rejection [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
- Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [seeWarnings and Precautions (5.2)].
Complications of Allogeneic HSCT
- Advise patients of the risk of post-allogeneic hematopoietic stem cell transplantation complications [seeWarnings and Precautions (5.3)].
Embryo-Fetal Toxicity:
- Advise females of reproductive potential that penpulimab-kcqx can cause harm to a fetus and to inform their healthcare provider of a known or suspected pregnancy [seeWarnings and Precautions (5.4)andUse in Specific Populations (8.1 , 8.3 )].
- Advise females of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of penpulimab-kcqx [seeUse in Specific Populations (8.3)].
Lactation:
- Advise female patients not to breastfeed while taking penpulimab-kcqx and for 4 months after the last dose [seeWarnings and Precautions (5.4)andUse in Specific Populations (8.2) ].
Manufactured by:
Akeso Biopharma Co., Ltd.
6 Shennong Road, Torch Development Zone, Zhongshan, Guangdong 528437, China
U.S. license number xxxx
|
MEDICATION GUIDE PENPULIMAB-KCQX (pen pul i mab kcqx) injection |
|||||||
|
What is the most important information I should know about penpulimab-kcqx? Penpulimab-kcqx is a medicine that may treat non-keratinizing nasopharyngeal cancer by working with your immune system. Penpulimab-kcqx can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended. Call or see your healthcare provider right away if you develop any new or worsening signs or symptoms, including: Lung problems |
|||||||
|
|
|
|||||
|
Intestinal problems
Liver problems |
|||||||
|
|
||||||
|
|
||||||
|
|||||||
|
Hormone gland problems |
|||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|||||||
|
Kidney problems |
|||||||
|
|
||||||
|
|
||||||
|
Skin problems |
|||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
Problems can also happen in other organs and tissues. These are not all of the signs and symptoms of immune system problems that can happen with penpulimab-kcqx. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
|
|||||||
|
Infusion reactions that can sometimes be severe or life-threatening. Signs and symptoms of infusion reactions may include: |
|||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
Rejection of a transplanted organ or tissue. Your healthcare provider should tell you what signs and symptoms you should report and monitor you depending on the type of organ or tissue transplant that you have had. Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with penpulimab-kcqx. Your healthcare provider will monitor you for these complications. Getting medical treatment right away may help keep these problems from becoming more serious. Your healthcare provider will check for these problems during treatment with penpulimab-kcqx. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with penpulimab-kcqx if you have severe side effects. |
|||||||
|
What is penpulimab-kcqx? Penpulimab-kcqx is a prescription medicine used to treat adults with a kind of cancer called non-keratinizing nasopharyngeal carcinoma (NPC).
|
|||||||
|
Before receiving penpulimab-kcqx, tell your healthcare provider about all of your medical conditions, including if you:
Females who are able to become pregnant:
|
|||||||
|
How will I receive penpulimab-kcqx?
|
|||||||
|
What are the possible side effects of penpulimab-kcqx? Penpulimab-kcqx can cause serious side effects. See “What is the most important information I should know about penpulimab-kcqx?” The most common side effects of penpulimab-kcqx when used in combination with either cisplatin or carboplatin and gemcitabine include: |
|||||||
|
|
|
|||||
|
|
|
|||||
|
|
| |||||
|
| ||||||
|
The most common side effects of penpulimab-kcqx when used alone include low red blood cell count (anemia) and low thyroid hormone levels. These are not all the possible side effects of penpulimab-kcqx. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||||
|
General information about the safe and effective use of penpulimab-kcqx. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about penpulimab-kcqx that is written for healthcare professionals. |
|||||||
|
What are the ingredients in penpulimab-kcqx? Active ingredient: penpulimab-kcqx Inactive ingredients: acetic acid, polysorbate 80, sodium acetate, sorbitol, and Water for Injection. Manufactured by: Akeso Biopharma Co., Ltd., 6 Shennong Road, Torch Development Zone, Zhongshan, Guangdong 528437, China U.S. License No. xxxx For more information, call 833-662-5376 or go to https://www.akesobio.com/en/. |
|||||||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: April/2025
| PENPULIMAB
KCQX
penpulimab injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akeso Biopharma, Co., Ltd (544314741) |
| Registrant - Akeso Biopharma, Co., Ltd (544314741) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akeso Biopharma, Co., Ltd | 544314741 | analysis(83654-105) , manufacture(83654-105) , pack(83654-105) , label(83654-105) | |
Penpulimab Biosimilars
Biosimilar and interchangeable products are biological products that are highly similar to and have no clinically meaningful differences from the reference product.
Reference products
These are biological products that have already been approved by the FDA, against which biosimilar products are compared. There is 1 for penpulimab.
More about penpulimab
- penpulimab consumer information
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: anti-PD-1 and PD-L1 monoclonal antibodies (immune checkpoint inhibitors)