Isopto Hyoscine Ophthalmic Solution: Package Insert / Prescribing Info
Package insert / product label
Generic name: scopolamine hydrobromide
Dosage form: ophthalmic solution
Drug class: Mydriatics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
Isopto Hyoscine Ophthalmic Solution Description
ISOPTO® Hyoscine (scopolamine hydrobromide ophthalmic solution) is an anticholinergic prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure:
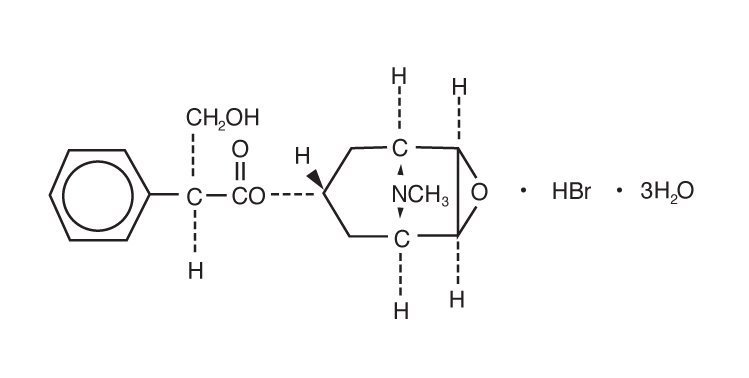
Established name: scopolamine hydrobromide
Chemical name: benzeneacetic acid, α-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]non-7-yl ester, hydrobromide, trihydrate, [7(S)-(1α,2β,4β,5α,7β)]-.
Each mL contains: Active: scopolamine hydrobromide 0.25%. Preservative: benzalkonium chloride 0.01%. Vehicle: hypromellose 0.5%. Inactives: sodium chloride, glacial acetic acid, sodium acetate (to adjust pH), purified water.
Isopto Hyoscine Ophthalmic Solution - Clinical Pharmacology
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
Indications and Usage for Isopto Hyoscine Ophthalmic Solution
For mydriasis and cycloplegia in diagnostic procedures. For some pre- and postoperative states when a mydriatic and cycloplegic is needed in treatment of iridocyclitis.
Contraindications
Contraindicated in persons with primary glaucoma or a tendency toward glaucoma, e.g., narrow anterior chamber angle; and in those showing hypersensitivity to any component of this preparation.
Warnings
Do not touch dropper tip to any surface, as this may contaminate the solution.
FOR TOPICAL OPHTHALMIC USE ONLY - NOT FOR INJECTION.
Precautions
To avoid excessive absorption, the lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. To avoid inducing angle closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made.
Patient Warning
Patient should be advised not to drive or engage in other hazardous activities when drowsy or while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child’s mouth and to wash their own hands and the child’s hands following administration.
Adverse Reactions/Side Effects
Prolonged use may produce local irritation, characterized by follicular conjunctivitis, vascular congestion, edema, exudate, and an eczematoid dermatitis. Somnolence, dryness of the mouth, or visual hallucinations may occur.
Related/similar drugs
Isopto Hyoscine Ophthalmic Solution Dosage and Administration
For refraction, administer one or two drops in the eye(s) one hour before refracting. For uveitis, administer one or two drops in the eye(s) up to four times daily.
How is Isopto Hyoscine Ophthalmic Solution supplied
In 5 mL plastic DROP-TAINER® dispensers.
5 mL NDC 0998-0331-05
| ISOPTO HYOSCINE
scopolamine hydrobromide solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Laboratories, Inc. | 008018525 | MANUFACTURE(0998-0331) | |
More about Isopto Hyoscine (scopolamine ophthalmic)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- Drug class: mydriatics
- Breastfeeding


