Eye Allergy Relief: Package Insert / Prescribing Info
Package insert / product label
Generic name: pheniramine maleate and naphazoline hydrochloride
Dosage form: ophthalmic solution
Drug class: Ophthalmic antihistamines and decongestants
Medically reviewed by Drugs.com. Last updated on Feb 27, 2025.
On This Page
Indications and Usage for Eye Allergy Relief
- •
- temporarily relieves itching and redness caused by pollen, ragweed, grass, animal hair and dander.
Warnings
For external use only
Do not use
- •
- if you are sensitive to any ingredient in this product
- •
- if solution changes color or becomes cloudy
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
- •
- narrow angle glaucoma
When using this product
- •
- overuse may cause more eye redness
- •
- pupils may become enlarged temporarily
- •
- do not touch tip of container to any surface to avoid contamination
- •
- you may feel a brief tingling after putting drops in eye
- •
- replace cap after use
- •
- remove contact lenses before using
Eye Allergy Relief Dosage and Administration
- •
- Adults and children 6 years of age and older: Instill 1 or 2 drops in the affected eye(s) up to 4 times daily.
- •
- Children under 6 years: ask a doctor
Related/similar drugs
Other information
- •
- store at 20°-25°C (68°-77°F)
- •
- this package is child-resistant
- •
- protect from light
- •
- use before expiration date marked on the carton or bottle
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, hypromellose, purified water, sodium borate, sodium chloride. Hydrochloric acid may be used to adjust pH.
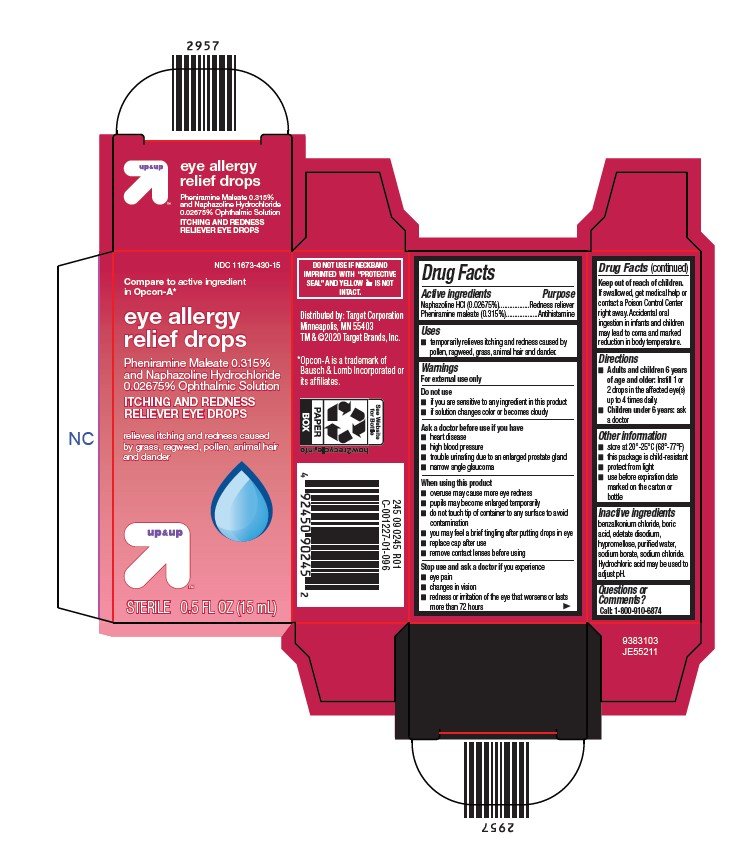
Package/Label Principal Display Panel
NDC 11673-430-15
Compare to active ingredient
in Opcon-A*
eye allergy
relief drops
Pheniramine Maleate 0.315%
and Naphazoline Hydrochloride
0.02675% Ophthalmic Solution
ITCHING AND REDNESS
RELIEVER EYE DROPS
relieves itching and redness caused
by grass, ragweed, pollen, animal hair
and dander
[drop icon]
[arrow icon] up & up™
STERILE 0.5 FL OZ (15 mL)
| EYE ALLERGY RELIEF
pheniramine maleate and naphazoline hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Target Corporation (006961700) |
| Registrant - Bausch & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 114406598 | MANUFACTURE(11673-430) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 079587625 | MANUFACTURE(11673-430) | |
More about Eye Allergy Relief (naphazoline / pheniramine ophthalmic)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- Drug class: ophthalmic antihistamines and decongestants