Copper: Package Insert / Prescribing Info
Package insert / product label
Generic name: cupric chloride
Dosage form: injection, solution
Drug class: Minerals and electrolytes
Medically reviewed by Drugs.com. Last updated on Jul 7, 2025.
On This Page
Copper Description
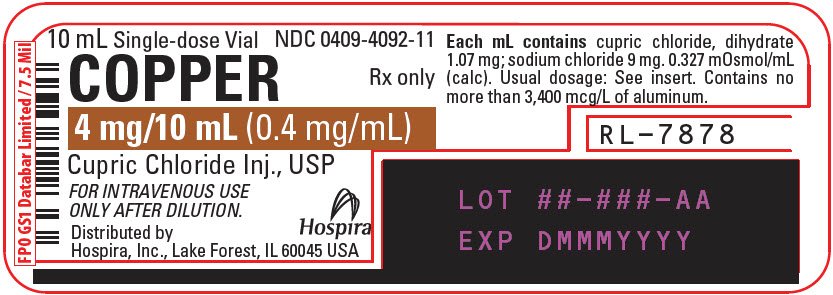
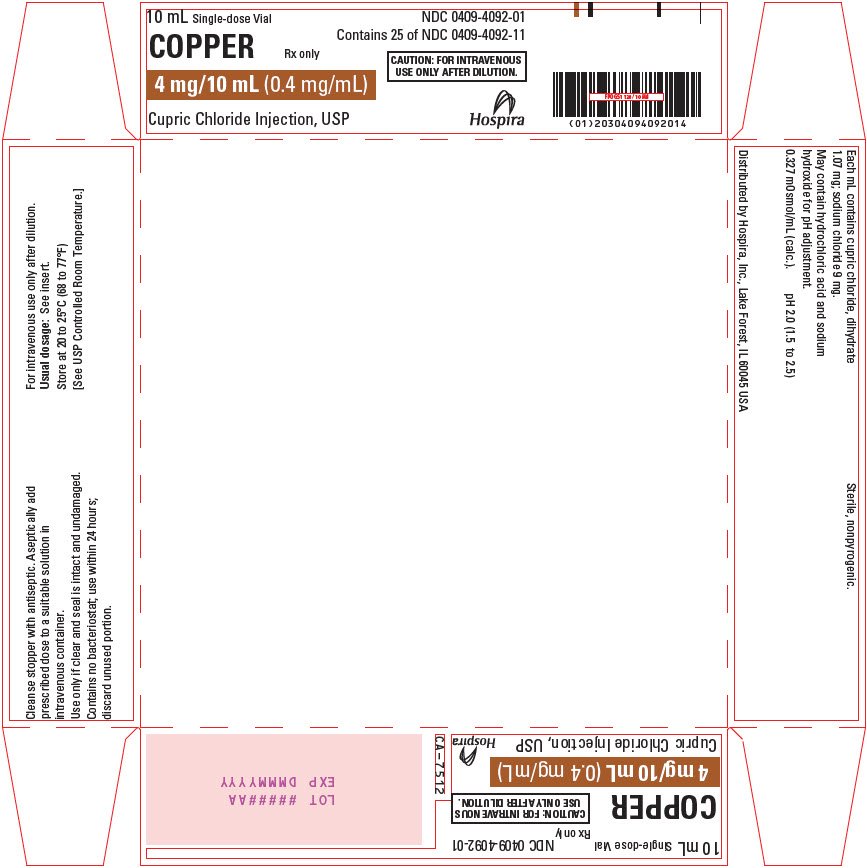
Copper (Cupric Chloride Injection, USP) 0.4 mg/mL is a sterile, nonpyrogenic solution intended for use as an additive to intravenous solutions for total parenteral nutrition (TPN). Each mL of solution contains 1.07 mg cupric chloride, dihydrate and 9 mg sodium chloride.
The solution contains no bacteriostat, antimicrobial agent or added buffer. The pH is 2.0 (1.5 to 2.5); product may contain hydrochloric acid and sodium hydroxide for pH adjustment. The osmolarity is 0.327 mOsmol/mL (calc.).
Cupric chloride, USP is chemically designated cupric chloride, dihydrate (CuCl2 • 2H2O), a crystalline compound freely soluble in water.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The small amount of water vapor that can pass through the plastic container wall will not significantly alter the drug concentration.
Copper - Clinical Pharmacology
Copper is an essential nutrient which serves as a cofactor for serum ceruloplasmin, an oxidase necessary for proper formation of the iron carrier protein, transferrin. Copper also helps maintain normal rates of red and white blood cell formation.
Providing copper during TPN helps prevent development of the following deficiency symptoms: Leukopenia, neutropenia, anemia, depressed ceruloplasmin levels, impaired transferrin formation, secondary iron deficiency and osteoporosis.
Normal serum copper values range from 80 to 163 mcg/dl (mean, approximately 110 mcg/dl). The serum copper level at which deficiency symptoms appear is not precisely defined.
In the plasma, about 7% of copper is bound to albumin and amino acids. In the liver, about 93% of copper is bound to ceruloplasmin and released to the serum. The daily turnover of copper through ceruloplasmin is approximately 0.5 mg. Copper is primarily excreted through the bile and into the gastrointestinal tract where it is not reabsorbed. Copper is also eliminated through the kidneys.
Indications and Usage for Copper
Copper (Cupric Chloride Injection) is indicated for use as a supplement to intravenous solutions given for TPN. Administration helps to maintain copper serum levels and to prevent depletion of endogenous stores and subsequent deficiency symptoms.
Contraindications
Copper (Cupric Chloride Injection) is contraindicated in patients with hypersensitivity to copper (see WARNINGS: Hypersensitivity Reactions).
Warnings
Hepatic Accumulation
Copper is primarily eliminated in the bile and excretion is decreased in patients with cholestasis and/or cirrhosis. Hepatic accumulation of copper has been reported in autopsies of patients receiving long-term parenteral nutrition containing copper at dosages higher than recommended.
Administration of copper to patients with cholestasis and/or cirrhosis may cause hepatic accumulation of copper. Administration of copper to patients with Wilson disease, an inborn error of copper metabolism with a defect in hepatocellular copper transport, may cause both increased hepatic accumulation of copper and aggravation of the underlying hepatocellular degeneration.
For patients with cholestasis, biliary dysfunction, or cirrhosis, monitor hepatic and biliary function during long-term administration of Cupric Chloride Injection. If a patient develops signs or symptoms of hepatobiliary disease during the use of Cupric Chloride Injection, obtain serum concentrations of copper and ceruloplasmin, and adjust the dose as indicated (see PRECAUTIONS: Hepatic Impairment).
Hypersensitivity Reactions
Postmarket safety reporting has identified copper hypersensitivity in women receiving copper-containing intrauterine devices, providing evidence that patients may experience hypersensitivity reactions when exposed to this metal. If hypersensitivity reactions (e.g., pruritis, angioedema, dyspnea, rash, urticaria) occur in patients receiving Cupric Chloride Injection in parenteral nutrition, discontinue the product, and initiate appropriate medical treatment (see CONTRAINDICATIONS).
Aluminum Toxicity
This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Precautions
Laboratory Tests
Twice monthly serum assays for copper and/or ceruloplasmin are suggested for monitoring copper concentrations in long-term TPN patients. As ceruloplasmin is a cuproenzyme, ceruloplasmin assays may be depressed secondary to copper deficiency.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of Copper 0.4 mg/mL (Cupric Chloride Injection) have not been performed, nor have studies been done to assess mutagenesis or impairment of fertility.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Copper 0.4 mg/mL (Cupric Chloride Injection) is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of Cupric Chloride Injection have been established in pediatric patients receiving parenteral nutrition (see DOSAGE AND ADMINISTRATION).
Pregnancy
Animal reproduction studies have not been conducted with cupric chloride. It is also not known whether cupric chloride can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Cupric chloride should be given to a pregnant woman only if clearly indicated.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Hepatic Impairment
Copper is primarily excreted in the bile. Excretion is decreased in patients with cholestasis and/or cirrhosis (see CLINICAL PHARMACOLOGY). Hepatic accumulation of copper has been reported with long-term administration of parenteral nutrition at dosages higher than recommended (see WARNINGS: Hepatic Accumulation). For patients with cholestasis or cirrhosis, monitor hepatic and biliary function during long-term administration of Cupric Chloride Injection. If a patient develops signs or symptoms of hepatobiliary disease during use of Cupric Chloride Injection, obtain serum concentrations of copper and ceruloplasmin.
Overdosage
Acute copper toxicity has been reported in patients with oral, intravenous, or subcutaneous administration. Clinical manifestations included metallic taste, nausea, vomiting, diarrhea, abdominal pain, neurological signs, such as encephalopathy, and multi-organ failure involving kidney, liver, blood, and cardiovascular systems, which may be fatal. Chelating agents, such as D-penicillamine, can be used for treatment of acute toxicity. Long-term administration of parenteral copper above the recommended dosage may result in significant accumulation of copper in the liver, brain, and other tissues with possible organ damage (see WARNINGS: Hepatic Accumulation).
Copper Dosage and Administration
- •
- Copper (Cupric Chloride Injection) contains 0.4 mg of copper per mL and is administered intravenously only after dilution. The additive should be diluted in a volume of fluid not less than 100 mL.
- •
- For the adult receiving TPN, the suggested additive dosage of copper is 0.3 to 0.5 mg/day.
- •
- For pediatric patients, the suggested additive dosage of copper is 20 mcg/kg/day (0.05 mL/kg/day) up to a maximum of 500 mcg/day.
- •
- This product is not appropriate for patients weighing less than 4 kg due to the inability to measure the appropriate amount of the product.
- •
- Do not administer Copper (Cupric Chloride Injection) intramuscularly because the acidic pH of the solution may cause considerable tissue irritation.
Copper (Cupric Chloride Injection) should only be used in conjunction with a pharmacy directed admixture program using aseptic technique in a laminar flow environment; it should be used promptly and in a single operation without any repeated penetrations. The solution contains no preservatives; discard the unused portion immediately after the admixture procedure is completed.
Copper (Cupric Chloride Injection) should be inspected visually for particulate matter and discoloration prior to administration. Do not use unless the solution is clear, and the seal is intact.
Cupric ion may degrade ascorbic acid in TPN solutions. In order to avoid this loss of ascorbate, multivitamin additives should be added to TPN solutions immediately prior to infusion. Alternatively, the multivitamin additive may be added to one container of TPN solution, followed by copper in a subsequent container.
How is Copper supplied
Copper 0.4 mg/mL (Cupric Chloride Injection, USP) is supplied as follows:
| Unit of Sale | Concentration |
|---|---|
|
NDC 0409-4092-01
|
4 mg/10 mL |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1067-3.0
Revised: 06/2025
| COPPER
cupric chloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 093132819 | ANALYSIS(0409-4092) , MANUFACTURE(0409-4092) , PACK(0409-4092) , LABEL(0409-4092) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 827731089 | ANALYSIS(0409-4092) | |