Budesonide (Ingredient)
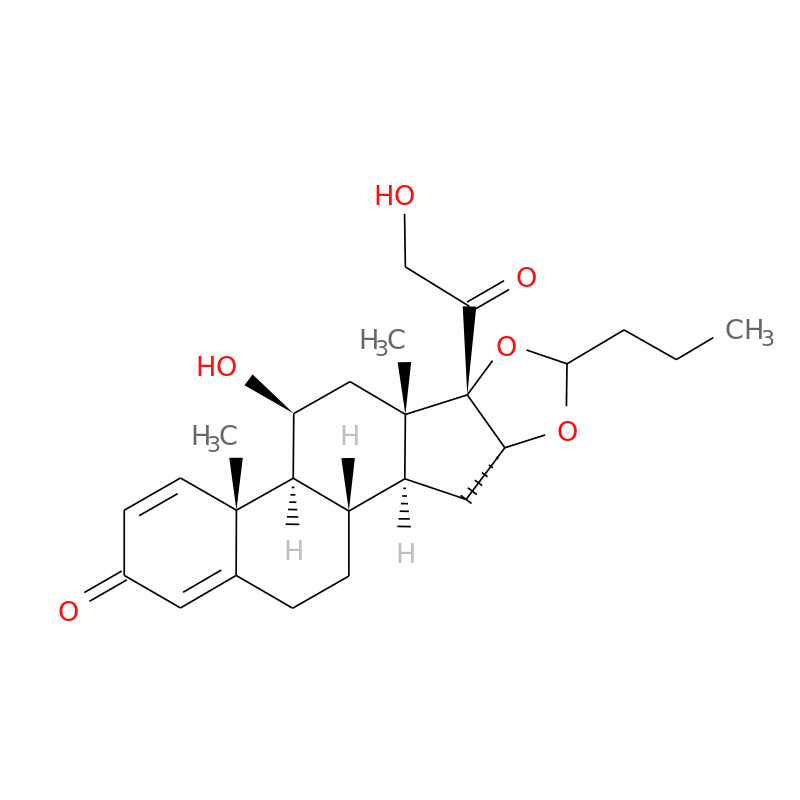
Chemical formula: C25H34O6
Drugbank ID: DB01222
ATC codes: R03AK07, A07EA06, R03BA02, R01AD05, D07AC09, R03AK12
The information below refers to products available in the United States that contain budesonide.
Products containing budesonide
budesonide systemic
Brand names: Pulmicort Flexhaler, Eohilia, Pulmicort Turbuhaler, Entocort EC, Uceris
Drug classes: glucocorticoids, inhaled corticosteroids
Budesonide systemic is used in the treatment of:
- Asthma
- Asthma, Maintenance
- Autoimmune Hepatitis
- Crohn's Disease
- Crohn's Disease, Active
- Crohn's Disease, Maintenance
- Eosinophilic Esophagitis
- Immunoglobulin A Nephropathy
- Inflammatory Bowel Disease
- Reversible Airways Disease, Maintenance
- Ulcerative Colitis
- Ulcerative Colitis, Active
budesonide nasal
Brand names: Rhinocort, Rhinocort Allergy, Rhinocort Aqua
Drug class: nasal steroids
Budesonide nasal is used in the treatment of:
Multi-ingredient medications containing budesonide
albuterol/budesonide systemic
Brand name: Airsupra
Drug class: antiasthmatic combinations
Albuterol/budesonide systemic is used in the treatment of:
budesonide/formoterol systemic
Brand names: Symbicort, Breyna
Drug class: bronchodilator combinations
Budesonide/formoterol systemic is used in the treatment of:
budesonide/formoterol/glycopyrrolate systemic
Brand name: Breztri Aerosphere
Drug class: bronchodilator combinations
Budesonide/formoterol/glycopyrrolate systemic is used in the treatment of:
Chemical structure
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
