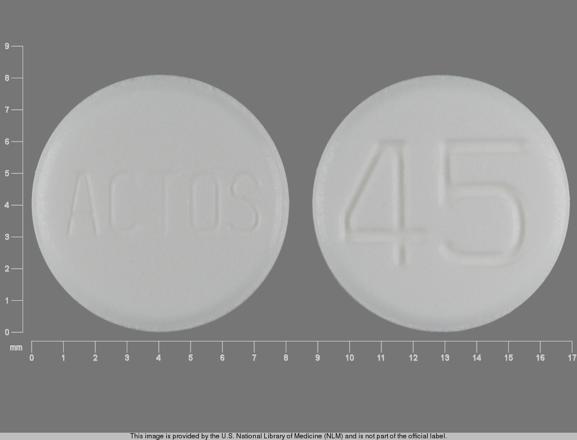
ACTOS 45 Pill: white, round, 8mm
Generic Name: pioglitazone
The pill with imprint ACTOS 45 (White, Round, 8mm) has been identified as Actos 45 mg and is used for Type 2 Diabetes. It belongs to the drug class thiazolidinediones and is not a controlled substance.
Images for ACTOS 45



Actos
- Generic Name
- pioglitazone
- Imprint
- ACTOS 45
- Strength
- 45 mg
- Color
- White
- Size
- 8.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Thiazolidinediones
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Takeda Pharmaceuticals America, Inc.
- Inactive Ingredients
-
carboxymethylcellulose calcium,
lactose monohydrate,
magnesium stearate
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00093-2046 (Discontinued) | Teva Pharmaceuticals USA, Inc. |
| 63304-0313 (Discontinued) | Ranbaxy Pharmaceuticals Inc. |
| 64764-0451 | Takeda Pharmaceutical Company Limited |
| 49999-0451 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
| 54569-4882 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 54868-4391 (Discontinued) | Physicians Total Care Inc. (repackager) |
| 68071-0407 | Nucare Pharmaceuticals Inc. (repackager) |
Related images for "ACTOS 45"
More about Actos (pioglitazone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (40)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: thiazolidinediones
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.