Vyjuvek Dosage
Generic name: BEREMAGENE GEPERPAVEC 5000000000[PFU] in 1mL;
Dosage form: topical suspension
Drug class: Miscellaneous topical agents
Medically reviewed by Drugs.com. Last updated on Jan 9, 2025.
For topical application on wounds only.
Dose
- The recommended dose of VYJUVEK gel is based on age (Table 1). VYJUVEK gel is applied topically to wound(s) once a week.
Table 1 Maximum Weekly Dose by Age
|
||
|
Age Range |
Maximum Weekly Dose (plaque forming units; PFU) |
Maximum Weekly Volume (milliliter; mL) * |
|
6 months to <3 years old |
1.6 ×10 9 |
0.8 |
|
≥ 3 years old |
3.2 ×10 9 |
1.6 |
- It may not be possible to apply VYJUVEK gel to all the wounds at each treatment visit.
- Apply VYJUVEK gel to wounds until they are closed before selecting new wound(s) to treat. Prioritize weekly treatment to previously treated wounds if they re-open. [ see Administration (2.3)]
- If a dose is missed, apply VYJUVEK gel as soon as possible and resume weekly dosing thereafter.
Preparation
Important Preparation Instructions
- Prepare VYJUVEK gel at the pharmacy by mixing the VYJUVEK biological suspension into the excipient gel for immediate use within 8 hours of application.
- Only a healthcare professional (HCP) should apply VYJUVEK gel either at a healthcare professional setting (e.g., clinic) or the home setting.
- Individuals who are pregnant should not prepare or apply VYJUVEK gel and should avoid direct contact with the treated wounds or dressings from treated wounds.
Below is the list of supplies needed for VYJUVEK gel preparation:
- One (1) carton containing one (1) VYJUVEK biological suspension vial and one (1) excipient gel vial ( Figure 1)
- Two (2) 18-gauge needles
- Two (2) to four (4) 1mL administration syringes
- One (1) 3mL preparation syringe
- Two (2) to four (4) syringe caps
- Protective gloves
- 70% isopropyl alcohol pads
- Biohazard container
- Labels for administration syringes
- Virucidal agent for clean-up
Follow the steps below for VYJUVEK gel preparation.
PREPARE THE PREPARATION SYRINGE
- Wash hands and put on protective gloves.
- Remove both vials from the carton and thaw the VYJUVEK biological suspension vial and the excipient gel vial at room temperature for AT LEAST 20 minutes ( Figure 1).
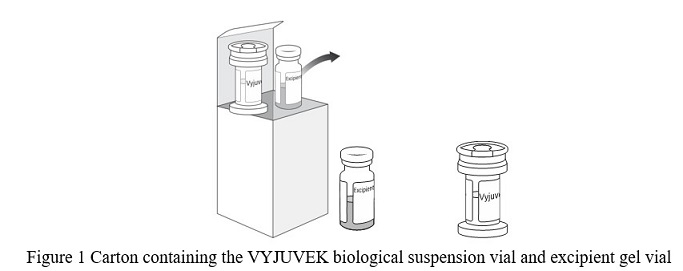
Note: Visually inspect the vials to ensure both are in liquid form and completely thawed. Excipient gel is more viscous and will take longer to thaw ( Figure 2).

Note: Once either the VYJUVEK biological suspension or the excipient gel is thawed, do not refreeze.
-
Invert the VYJUVEK biological suspension vial 4-5 times. Do not invert the excipient gel vial.
- Remove the caps from the vials and clean each vial stopper with a 70% isopropyl alcohol pad. Allow them to dry.
- Aseptically connect an 18-gauge needle to the 3 mL preparation syringe.
- Remove the needle cap and puncture the VYJUVEK biological suspension vial stopper.
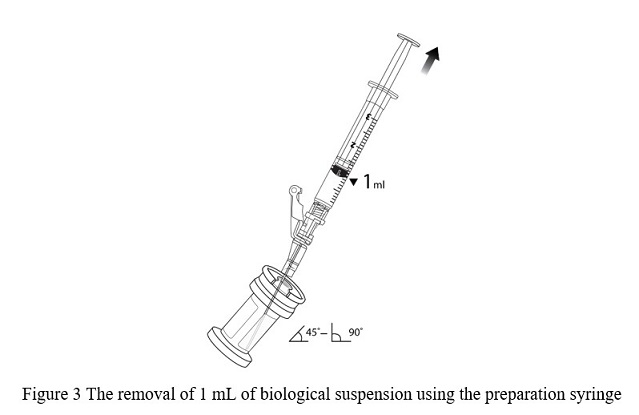
- Hold the vial at 45 to 90 degrees and withdraw 1 mL of VYJUVEK biological suspension into the preparation syringe ( Figure 3).
- Remove the preparation syringe (still connected to the needle) containing 1 mL of VYJUVEK biological suspension from the vial. Do NOT engage the safety lock.
- Discard the VYJUVEK biological suspension vial in the biohazard waste.
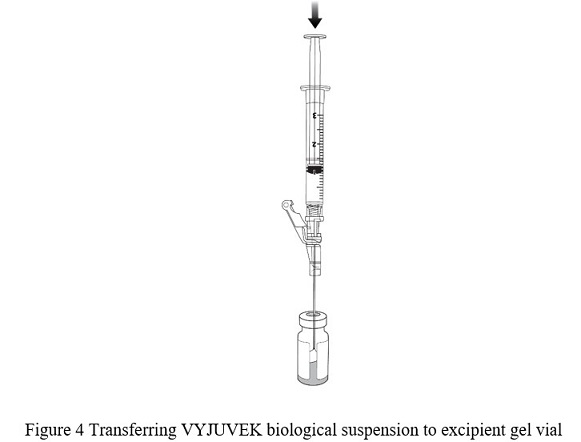
- Puncture the clean excipient gel stopper and transfer the VYJUVEK biological suspension into the excipient gel vial ( Figure 4).
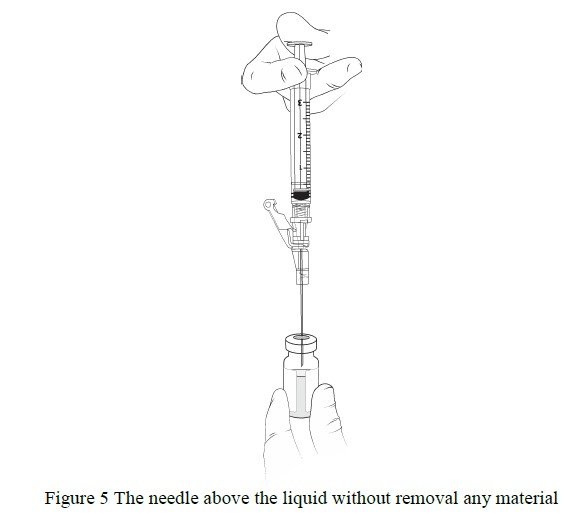
- WITHOUT REMOVING THE NEEDLE from the excipient gel vial, lift the bevel of the needle above the liquid ( Figure 5)and pull the plunger back to the 1 mL mark ( Figure 6).
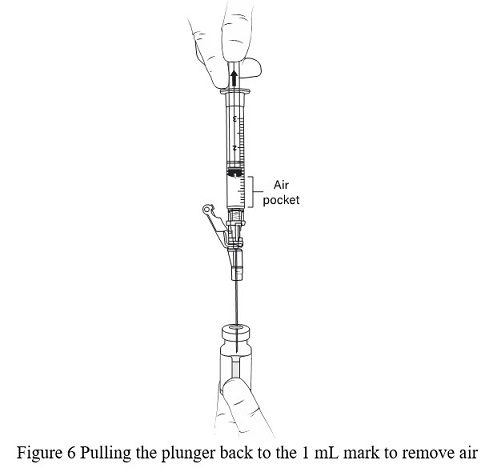
- Remove the preparation syringe with the 1 mL of air and engage the safety lock.
- Discard the preparation syringe and needle into the biohazard waste.
- Place a 70% isopropyl alcohol pad on top of the excipient gel stopper and hold it tightly in place.
- Shake VIGORIOUSLY for 10 SECONDS ( Figure 7).
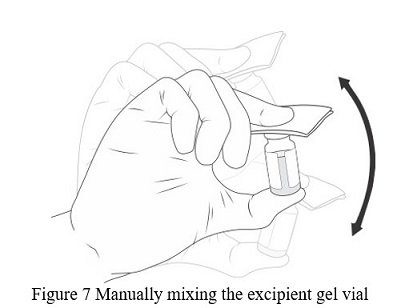
Note: The mixture of VYJUVEK biological suspension and excipient gel is referred to as VYJUVEK gel.
PREPARE THE ADMINISTRATION SYRINGES
- Aseptically connect an 18-gauge needle to the first 1 mL administration syringe and remove the needle cap.
- Insert the 18-gauge needle into the excipient vial containing VYJUVEK gel ( Figure 8).

- Tilt the vial 45 to 90 degrees and withdraw 0.4 mL of VYJUVEK gel ( Figure 9).
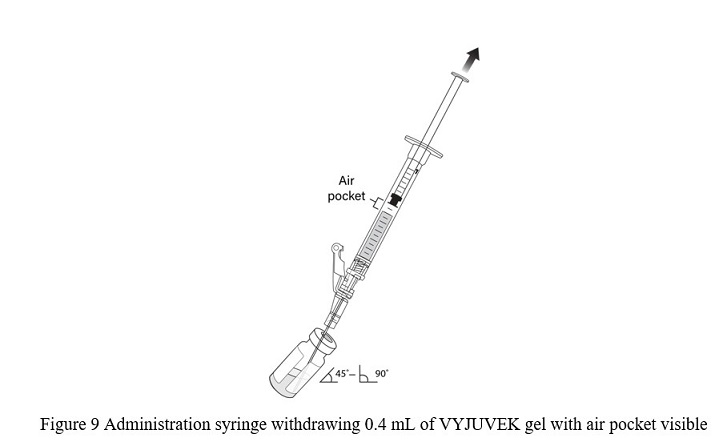
Note: An air pocket may form near the plunger when extracting VYJUVEK gel.
- DO NOT REMOVE THE NEEDLE FROM THE VIAL; lift the bevel of the needle above the VYJUVEK gel and disconnect the administration syringe containing 0.4 mL of mixed VYJUVEK ( Figure 10).
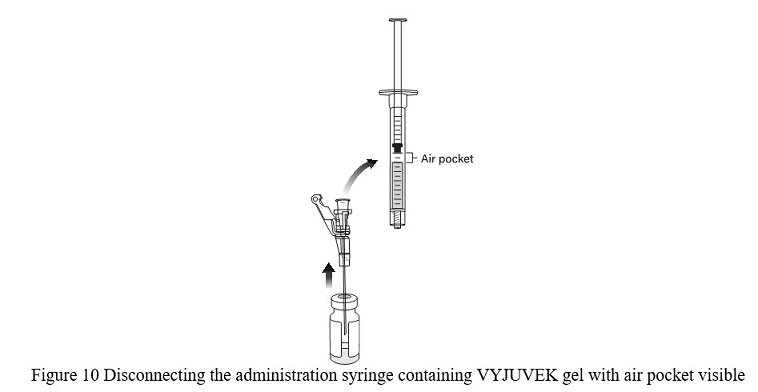
Note: Leave the needle in the excipient gel vial stopper.
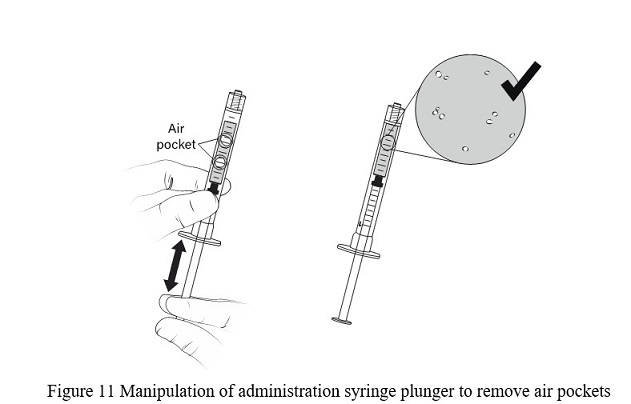
- DO NOT flick the syringe to remove the air pocket. Manipulate the plunger up and down, until all air pockets have been removed ( Figure 11)
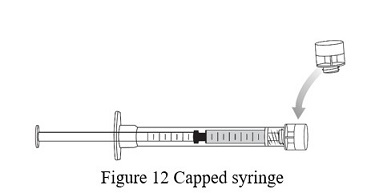
- Cap the administration syringe and set aside ( Figure 12).
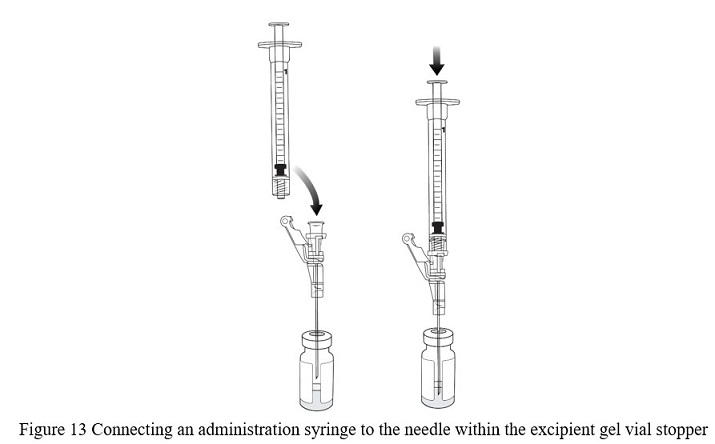
- Connect a new 1 mL administration syringe to the needle that remains in the excipient gel vial stopper (with the bevel of the needle ABOVE the VYJUVEK gel) ( Figure 13).
- Place the bevel of the needle in the VYJUVEK gel, tilt the vial 45 to 90 degrees and withdraw 0.4 mL of VYJUVEK gel into the administration syringe.
- Complete steps 19, 20, and 21 above to disconnect the administration syringe and remove the air pockets, prior to capping the administration syringe ( Figure 10, Figure 11, and Figure 12).
- Repeat steps 22, 23, and 24 if two (2) additional administration syringes are required (each containing 0.4 mL of VYJUVEK gel).
Note: Administration syringes are labeled as syringe #1, #2, #3, and #4.
- Discard the excipient gel vial (with the needle within the vial stopper) into the biohazard container.
- Clean all surfaces that may have come in contact with VYJUVEK biological suspension or gel and treat all spills with a virucidal agent such as 70% isopropyl alcohol, 6% hydrogen peroxide or <0.4% ammonium chloride. Blot using absorbent materials.
- Dispose all materials (e.g., vial, syringe, needle, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
- Place the capped administration syringes containing the VYJUVEK gel in a sealable plastic bag.
- Place the sealable plastic bag with administration syringes into an appropriate insulated secondary container at 2º to 8ºC (35.6º to 46.4ºF) for transport from the preparation site to the administration site.
Administration
Below is the list of supplies needed for VYJUVEK gel administration:
- The administration syringes
- Non-adherent hydrophobic dressing
- Scissors
- Standard dressing
- Protective gloves
- Biohazard container
- Virucidal agent for clean-up
Use the VYJUVEK gel in the administration syringes immediately after preparation. If immediate use is not possible, please refer to section [ seeStorage and Handling(16.2)].
VYJUVEK GEL ADMINISTRATION
Follow the steps below for VYJUVEK gel administration.
- Apply VYJUVEK gel to the selected wound(s) in droplets spaced evenly within the wound, approximately 1cm-by-1cm apart. The resulting droplet pattern should loosely resemble a grid. Avoid touching the administration syringe to the skin ( Figure 14).
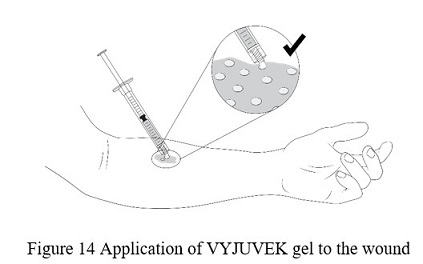
Table 2below provides a reference on dose per approximate size of the wound.
Table 2 by Wound Size
* For wound area over 60 cm 2, recommend calculating the total dose based on Table 2 until the maximum weekly dose in Table 1is reached. Wound Area (cm 2) Dose (PFU) Volume (mL) < 20
4×10 8
0.2
20 to < 40
8×10 8
0.4
40 to 60
1.2×10 9
0.6
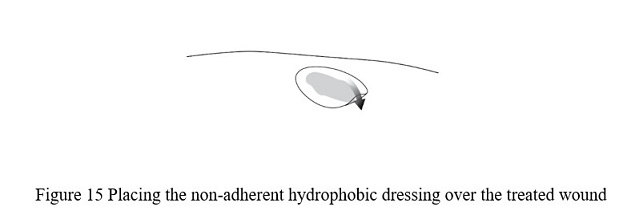
- Use the clean scissors to cut the non-adherent hydrophobic dressing to a size slightly larger than the wound and place the dressing atop the VYJUVEK gel droplets ( Figure 15).
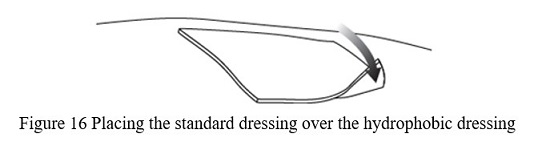
- Use the scissors to cut the standard dressing used by the patient to a size slightly larger than the hydrophobic dressing and place the standard dressing atop the hydrophobic dressing ( Figure 16).
- Clean all surfaces that may have come in contact with VYJUVEK gel and treat all spills with a virucidal agent such as 70% isopropyl alcohol, 6% hydrogen peroxide or <0.4% ammonium chloride. Blot using absorbent materials.
- Dispose all materials (e.g., syringe, cleaning materials) that may have come in contact with VYJUVEK biological suspension or gel into a biohazard bag or container.
- Discard unused administration syringes containing the VYJUVEK gel after preparation into a biohazard bag or container [ see Storage and Handling (16.2)] .
- Do not change wound dressing within approximately 24 hours after VYJUVEK gel application.
More about Vyjuvek (beremagene geperpavec topical)
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous topical agents
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.