Pombiliti Dosage
Generic name: CIPAGLUCOSIDASE ALFA 105mg
Dosage form: injection, powder, lyophilized, for solution
Drug class: Lysosomal enzymes
Medically reviewed by Drugs.com. Last updated on Nov 27, 2024.
Pregnancy Evaluation Prior to Initiating Treatment
Verify the pregnancy status of females of reproductive potential prior to initiating POMBILITI in combination with Opfolda.
Recommended Dosage and Administration
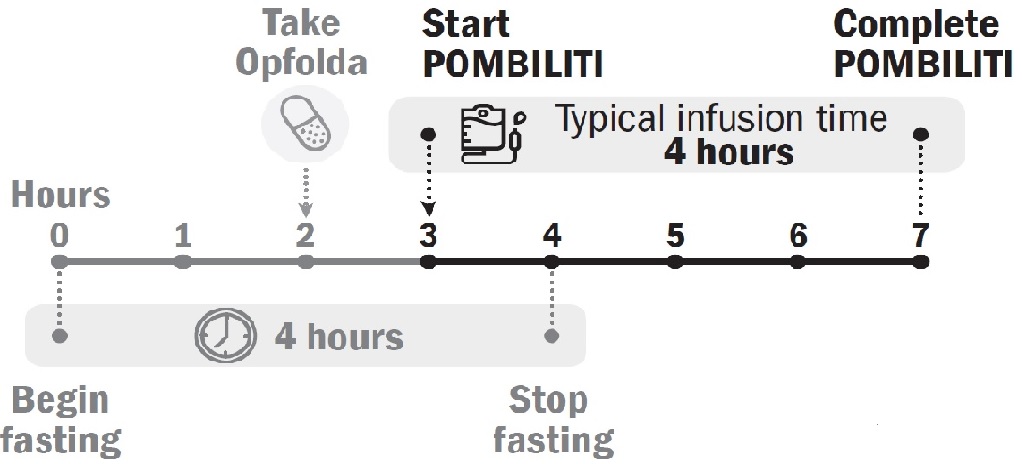
POMBILITI must be administered in combination with Opfolda (see Figure 1 for the dosing timeline). If the Opfolda dose is missed, POMBILITI should not be administered. Refer to the Opfolda Prescribing Information for Opfolda dosage and administration recommendations.
Prior to POMBILITI administration, consider pretreating with antihistamines, antipyretics, and/or corticosteroids. If premedication was used with previous enzyme replacement therapy (ERT), prior to POMBILITI administration, pretreat with antihistamines, antipyretics, and/or corticosteroids.
The recommended dosage of POMBILITI is 20 mg/kg (of actual body weight) administered every other week as an intravenous infusion over approximately 4 hours (see Table 1 for the recommended total infusion volume based on the patient’s weight).
Start POMBILITI in combination with Opfolda 2 weeks after the last ERT dose.
Initiate the POMBILITI infusion approximately 1 hour after oral administration of Opfolda. If the POMBILITI infusion cannot be started within 3 hours of oral administration of Opfolda, reschedule POMBILITI in combination with Opfolda at least 24 hours after Opfolda was last taken. If POMBILITI in combination with Opfolda are both missed, re-start treatment as soon as possible.
Dosage and Administration Modifications Due to Hypersensitivity Reactions and/or Infusion-Associated Reactions
In the event of a severe hypersensitivity reaction (including anaphylaxis) or a severe infusion-associated reaction (IAR), immediately discontinue the POMBILITI infusion, and initiate appropriate medical treatment. For additional recommendations in the event of a severe hypersensitivity reaction.
In the event of a mild to moderate hypersensitivity reaction or moderate IAR, consider temporarily holding or slowing the infusion rate and initiating appropriate medical treatment. If symptoms:
- Persist despite temporarily holding or slowing the infusion, stop the infusion for 30 to 60 minutes, monitor the patient, and consider resuming the infusion at a reduced rate if symptoms have improved. If symptoms continue to persist, discontinue the infusion, and consider re-initiating the infusion within 7 to 14 days with appropriate premedication.
- Subside following holding or slowing the infusion, increase the infusion rate to the rate at which the reaction occurred and consider continuing to increase the rate (every 30 minutes) in a stepwise manner up to the target infusion rate. Closely monitor the patient.
Reconstitution and Dilution Instructions
Use aseptic technique during preparation. Reconstitute and dilute POMBILITI in the following manner:
Reconstitute the Lyophilized Powder
- Determine the number of POMBILITI vials to be reconstituted based on the calculated dose (based on patient’s actual body weight in kg).
- Remove vials from the refrigerator and set aside for approximately 30 minutes to allow vials to come to room temperature.
- Reconstitute each vial by slowly injecting 7.2 mL of Sterile Water for Injection, down the inside wall of each vial to avoid foaming. Avoid forceful impact of Sterile Water for Injection on the lyophilized powder and avoid foaming.
- Roll and tilt each vial to allow the lyophilized powder to dissolve completely which typically takes 2 minutes. Each vial will yield a concentration of 15 mg/mL. Do not invert, swirl, or shake.
- Visually inspect the reconstituted solution for particulate matter and discoloration. The reconstituted solution appears as a clear to opalescent, colorless to yellowish solution essentially particle free. Discard if foreign matter is observed or the solution is discolored.
Dilute the Reconstituted Solution
- Remove airspace within a bag of 0.9% Sodium Chloride Injection. Remove an equal volume of 0.9% Sodium Chloride Injection from the bag that will be replaced by the total volume (mL) of reconstituted POMBILITI (see Table 1 for the recommended total infusion volume based on the patient’s weight).
- Slowly withdraw 7 mL of reconstituted solution from each of the vials until the patient’s dose is obtained. Discard any remaining reconstituted solution in the last vial.
- Add the reconstituted solution slowly and directly into the infusion bag.
- To prevent foaming, gently invert infusion bag to mix the solution and avoid vigorous shaking or agitation. After dilution, the solution will have a final concentration of 0.5 to 4 mg/mL of cipaglucosidase alfa-atga. Do not use a pneumatic tube to transport the infusion bag.
- Administer the diluted solution at room temperature without delay.
Storage Instructions for the Reconstituted and Diluted Product
Storage of the Reconstituted Solution
If the reconstituted POMBILITI vials are not used immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours. Do not freeze.
Storage of the Diluted Solution
If the diluted solution is not administered immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) for up to 16 hours. Storage at room temperature is not recommended. Do not freeze.
After removal of the diluted solution from the refrigerator:
- Completely infuse within 6 hours.
- Do not restore in the refrigerator.
Discard the diluted solution if refrigerated more than 16 hours or if the diluted solution is not able to be completely infused within 6 hours after removal from the refrigerator.
Administration Instructions
Prior to administration, inspect the infusion bag for foaming. If foaming is present, let foam dissipate before administering POMBILITI. If the diluted solution has been refrigerated, allow solution to equilibrate to room temperature for 30 minutes prior to infusion.
Use an administration set with an inline low protein binding 0.2‑micron filter to administer POMBILITI. Change filter if the filter becomes blocked.
Initiate the POMBILITI infusion approximately 1 hour after oral administration of Opfolda. In the event of POMBILITI infusion delay, the starting infusion time should not exceed 3 hours from the oral administration of Opfolda.
The initial recommended infusion rate is 1 mg/kg/hour (see Table 1). Gradually increase the infusion rate by 2 mg/kg/hour every 30 minutes if there are no signs of hypersensitivity or infusion-associated reactions (IARs) until a maximum rate of 7 mg/kg/hour is reached; then, maintain the infusion rate at 7 mg/kg/hour until the infusion is complete. The approximate total infusion duration is 4 hours.
See Table 1 for the rate of infusion at each step, expressed as mL/hour based on the recommended infusion volume by patient weight.
Do not infuse POMBILITI in the same intravenous line with other products.
Table 1. Recommended POMBILITI Infusion Volumes and Rates by Patient Weight
| Patient Weight Range | Total Infusion Volume | Step 1 1 mg/kg/hour |
Step 2 3 mg/kg/hour |
Step 3 5 mg/kg/hour |
Step 4 7 mg/kg/hour |
| Infusion Rate in mL/hour | |||||
| 40–50 kg | 250 mL | 13 | 38 | 63 | 88 |
| 50.1–60 kg | 300 mL | 15 | 45 | 75 | 105 |
| 60.1-100 kg | 500 mL | 25 | 75 | 125 | 175 |
| 100.1–120 kg | 600 mL | 30 | 90 | 150 | 210 |
| 120.1–140 kg | 700 mL | 35 | 105 | 175 | 245 |
More about Pombiliti (cipaglucosidase alfa)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: lysosomal enzymes
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.