Arexvy Dosage
Generic name: RECOMBINANT RESPIRATORY SYNCYTIAL VIRUS PRE-FUSION F PROTEIN ANTIGEN 120ug in 0.5mL;
Dosage form: injection kit
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Aug 8, 2025.
Preparation
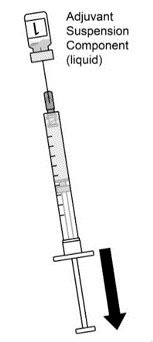
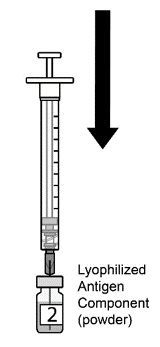
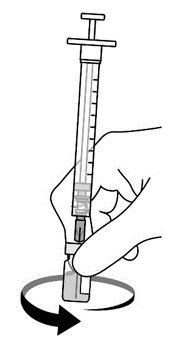
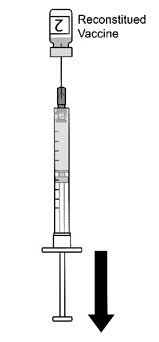
AREXVY is supplied in 2 vials that must be combined prior to administration. Prepare AREXVY by reconstituting the lyophilized antigen component (a sterile white powder) with the accompanying adjuvant suspension component (an opalescent, colorless to pale brownish sterile liquid). Use only the supplied adjuvant suspension component for reconstitution. The reconstituted vaccine should be an opalescent, colorless to pale brownish liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
Frequently asked questions
- What is the difference between Arexvy and Abrysvo?
- How many doses of Arexvy are required?
- What are the symptoms of RSV(respiratory syncytial virus)
- Is Arexvy a live vaccine?
- What kind of vaccine is Arexvy?
More about Arexvy (rsv vaccine pref3, recombinant)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Latest FDA alerts (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: viral vaccines
- En español
Patient resources
- Arexvy drug information
- Arexvy (Respiratory syncytial virus vaccine, adjuvanted Intramuscular) (Advanced Reading)
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.