Adzynma Dosage
Generic name: Apadamtase Alfa 500[iU] in 1[iU];
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Aug 30, 2024.
For intravenous use after reconstitution only.
Dosage
- Each vial of ADZYNMA is labeled with the actual rADAMTS13 activity, measured in terms of its potency in International Units (IU).
- Calculate administration dose and volume based on the patient's body weight using the actual potency (and not the nominal potency) as printed on ADZYNMA vial.
- For Intravenous (IV) Infusion at a rate of 2 to 4 mL per minute.
Prophylactic Therapy
The recommended prophylactic dosage regimen of ADZYNMA is as follows:
- Administer 40 IU/kg body weight once every other week.
- The prophylactic dosing frequency may be adjusted to 40 IU/kg body weight once weekly based on prior prophylactic dosing regimen or clinical response.
On-Demand Therapy
A guide for dosing ADZYNMA for on demand treatment of an acute event is provided in Table 1.
Table 1: Dosing for On-Demand Therapy
| Treatment Day 1 |
Treatment Day 2 |
Treatment Day 3 and Beyond |
| 40 IU/kg |
20 IU/kg |
15 IU/kg once daily until two days after the acute event is resolved. |
Preparation and Administration
- Use aseptic technique (clean and germ-free) throughout the procedure.
- Check the expiration date of the product prior to use.
- Do not use ADZYNMA if the expiration date has passed.
Reconstitution
- 1.
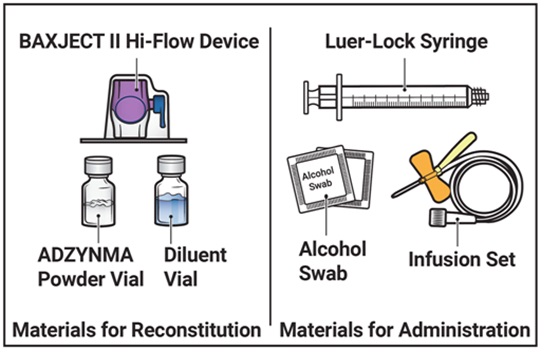
- Prepare a clean, germ-free, flat surface and gather all the materials you will need for the reconstitution and infusion. Figure A depicts the materials provided in the carton box.
Figure A
 |
- 2.
- Do not use ADZYNMA if the expiration date has passed.
- Use ADZYNMA within 3 hours after reconstitution and keep at room temperature not to exceed 86°F/30°C. Do not store at any other temperature. Discard any unused reconstituted product if not used within 3 hours after reconstitution.
- 3.
- Allow the vials of ADZYNMA and diluent to reach room temperature before use.
- If the patient needs more than one vial of ADZYNMA per injection, reconstitute each vial according to the instructions stated under ‘Reconstitution’.
- Inspect the reconstituted ADZYNMA solution for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance.
- Do not administer if particulate matter or discoloration is observed.
- 4.
- Wash and dry your hands thoroughly, and put on clean exam gloves.
- 5.
- Remove plastic caps from the ADZYNMA and diluent vials and place the vials on a flat surface (Figure B).
Figure B
 |
- 6.
- Wipe the rubber stoppers with an alcohol swab and allow them to dry prior to use (Figure C).
Figure C
 |
- 7.
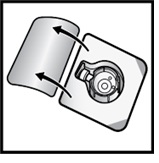
- Open the BAXJECT II Hi-Flow device package by peeling away the lid, without touching the inside (Figure D).
- Do not remove the BAXJECT II Hi-Flow device from the package.
- Do not touch the clear plastic spike.
Figure D
 |
- 8.
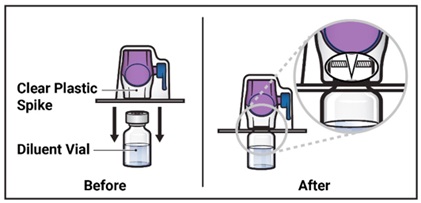
- Turn the package with the BAXJECT II Hi-Flow device upside down and place it over the top of the diluent vial. Press straight down until the clear plastic spike pierces through the diluent vial stopper (Figure E).
Figure E
 |
- 9.
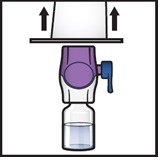
- Grip the BAXJECT II Hi-Flow device package at its edge and pull the package off the device (Figure F).
- Do not remove the blue cap from the BAXJECT II Hi-Flow device.
- Do not touch the exposed purple plastic spike.
Figure F
 |
- 10.
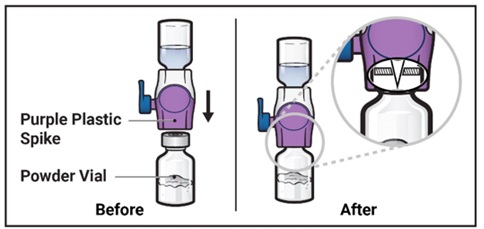
- Turn the system over so that the diluent vial is now on top. Press the BAXJECT II Hi-Flow device straight down until the purple plastic spike pierces through the ADZYNMA powder vial stopper (Figure G). The vacuum will draw the diluent into the ADZYNMA powder vial.
- You may notice some bubbles or foam – this is normal and should soon disappear. Wait until foam or bubbles dissipate before administration.
Figure G
 |
- 11.
- Swirl the connected vials gently and continuously until the powder is completely dissolved (Figure H).
Figure H
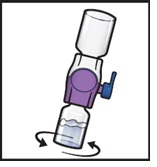 |
- 12.
- Visually inspect the reconstituted solution for particulate matter before administration. The solution should be clear and colorless in appearance.
- Do not use the product if particulate matter or discoloration is observed.
- 13.
- If the dose requires more than one vial of ADZYNMA, repeat step 1 to step 12 to reconstitute each vial.
- Use a different BAXJECT II Hi-Flow device to reconstitute each vial of ADZYNMA and diluent.
Administration Instructions
- 14.
- Take off the blue cap from the BAXJECT II Hi-Flow device (Figure I). Attach a Luer-lock syringe (Figure J).
- Do not inject air into the system.
Figure I
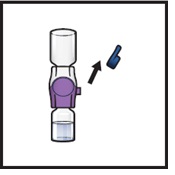 |
Figure J
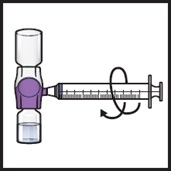 |
- 15.
- Turn the system upside down (ADZYNMA vial is now on top). Draw the reconstituted solution into the syringe by pulling the plunger back slowly (Figure K).
- Use ADZYNMA within 3 hours after reconstitution and keep at room temperature not to exceed 86°F/30°C. Do not store at any other temperature. Discard any unused reconstituted product if not used within 3 hours after reconstitution.
- Do not administer ADZYNMA in the same tubing or container at the same time with other medicinal products for infusion.
Figure K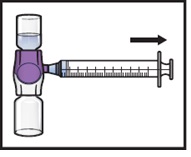 |
- 16.
- If a patient is to receive more than one vial of ADZYNMA, the contents of multiple vials can be drawn into the same syringe. Repeat this process for all reconstituted vials of ADZYNMA until the total volume to be administered is reached.
- 17.
- Disconnect the syringe and attach a suitable injection needle or an infusion set.
- 18.
- Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
- 19.
- Apply a tourniquet and clean the chosen infusion site with an alcohol swab (Figure L).
Figure L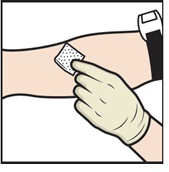 |
- 20.
- Insert the needle into the vein and remove the tourniquet.
- 21.
- Infuse the reconstituted ADZYNMA slowly, at a rate of 2 to 4 mL per minute (Figure M).
- A syringe pump may be used to control the rate of administration.
Figure M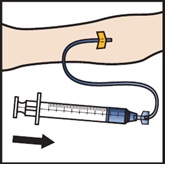 |
- 22.
- Take the needle out of the vein, place a cotton ball or gauze on the infusion site, and apply pressure for several minutes to stop bleeding.
- 23.
- Place the needle, syringe, and empty vials in a puncture-resistant sharps container.
- Do not dispose of syringes and needles in the regular waste.
Does Adzynma interact with my other drugs?
Enter medications to view a detailed interaction report using our Drug Interaction Checker.
More about Adzynma (apadamtase alfa, recombinant)
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Medical Disclaimer