Vusion: Package Insert / Prescribing Info
Package insert / product label
Generic name: miconazole nitrate, zinc oxide, white petrolatum
Dosage form: ointment
Drug class: Topical antifungals
Medically reviewed by Drugs.com. Last updated on Feb 3, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
VUSION® (miconazole nitrate, zinc oxide, and white petrolatum) ointment, for topical use
Initial U.S. Approval: 2006
Indications and Usage for Vusion
- •
- VUSION Ointment is indicated for adjunctive treatment of diaper dermatitis when complicated by documented candidiasis (microscopic evidence of pseudohyphae and /or budding yeast) in immunocompetent pediatric patients 4 weeks and older. (1.1)
- •
- VUSION Ointment should not be used as a substitute for frequent diaper changes. (1.1)
- •
- VUSION Ointment should not be used to prevent the occurrence of diaper dermatitis, since preventative use may result in the development of drug resistance. (1.2)
Vusion Dosage and Administration
- •
- VUSION Ointment is for topical use only. VUSION Ointment is not for oral, ophthalmic, or intravaginal use. (2)
- •
- VUSION Ointment should be applied as a thin layer to the affected area at each diaper change for 7 days. (2)
- •
- VUSION Ointment should be used as part of a treatment regimen that includes gentle cleansing of the diaper area and frequent diaper changes. (2)
Dosage Forms and Strengths
- •
- Ointment with 0.25% miconazole nitrate, 15% zinc oxide, and 81.35% white petrolatum. (3)
Contraindications
- •
- None
Warnings and Precautions
- •
- If irritation occurs or if the disease worsens, discontinue use of the medication, and contact the health care provider. (5.1)
Adverse Reactions/Side Effects
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2018
Full Prescribing Information
1. Indications and Usage for Vusion
1.1 Indication
VUSION Ointment is indicated for the adjunctive treatment of diaper dermatitis only when complicated by documented candidiasis (microscopic evidence of pseudohyphae and/or budding yeast), in immunocompetent pediatric patients 4 weeks and older. A positive fungal culture for Candida albicans is not adequate evidence of candidal infection since colonization with C. albicans can result in a positive culture. The presence of candidal infection should be established by microscopic evaluation prior to initiating treatment.
VUSION should be used as part of a treatment regimen that includes measures directed at the underlying diaper dermatitis, including gentle cleansing of the diaper area and frequent diaper changes. VUSION should not be used as a substitute for frequent diaper changes.
1.2 Limitations of Use
The safety and efficacy of VUSION have not been demonstrated in immunocompromised patients, or in infants less than 4 weeks of age (premature or term).
The safety and efficacy of VUSION have not been evaluated in incontinent adult patients. VUSION should not be used to prevent the occurrence of diaper dermatitis, such as in an adult institutional setting, since preventative use may result in the development of drug resistance.
2. Vusion Dosage and Administration
VUSION is not for oral, ophthalmic, or intravaginal use.
Before applying VUSION, gently cleanse the skin with lukewarm water and pat dry with a soft towel. Avoid using any scented soaps, shampoos, or lotions on the diaper area.
Gently apply a thin layer of VUSION to the diaper area with each diaper change for 7 days. Do not rub VUSION into the skin as this may cause additional irritation. Thoroughly wash hands after applying VUSION. Continue treatment for the full 7 days, even if there is improvement.
Do not use VUSION for longer than 7 days. The safety of VUSION when used for longer than 7 days is not known. If symptoms have not improved by day 7, see your health care provider.
3. Dosage Forms and Strengths
VUSION (miconazole nitrate, zinc oxide and white petrolatum) contains 2.5 mg of miconazole nitrate USP, 150 mg of zinc oxide USP and 813.5 mg of white petrolatum USP per gram.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rate observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 835 infants and young children were evaluated in the clinical development program. Of 418 subjects in the VUSION group, 58 (14%) reported one or more adverse events. Of 417 subjects in the zinc oxide/white petrolatum control group, 85 (20%) reported one or more adverse events. Adverse events that occurred at a rate of ≥ 1% for subjects who were treated with VUSION were approximately the same in type and frequency as for subjects who were treated with zinc oxide/white petrolatum ointment.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post approval use of VUSION. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: vomiting
General Disorders and Administration Site Conditions: burning sensation, condition aggravated, inflammation, pain
Injury, Poisoning and Procedural Complications: accidental exposure
Skin and Subcutaneous Tissue Disorders: blister, dermatitis contact, diaper dermatitis, dry skin, erythema, pruritus, rash, skin exfoliation
Related/similar drugs
7. Drug Interactions
Drug-drug interaction studies were not conducted. Women who take a warfarin anticoagulant and use a miconazole intravaginal cream or suppository may be at risk for developing an increased prothrombin time, international normalized ratio (INR), and bleeding. The potential for this interaction between warfarin and VUSION is unknown.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on VUSION Ointment use in pregnant women to inform a drug‑associated risk for adverse developmental outcomes. In animal reproduction studies, prolonged gestation, increased number of resorptions, and decreased numbers of live young were observed after oral administration of miconazole nitrate during organogenesis to pregnant rats and rabbits. No comparisons of animal exposure with human exposure may be calculated due to minimal systemic exposure in humans after topical administration of VUSION [see Clinical Pharmacology (12.3)].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There is no available information on the presence of miconazole in human milk, or the effects on the breastfed child, or the effects on milk production following use of VUSION.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VUSION and any potential adverse effects on the breastfed infant from VUSION or from the underlying maternal condition.
8.4 Pediatric Use
Efficacy was not demonstrated in infants less than 4 weeks of age. Safety and efficacy have not been established in very-low-birth-weight infants (less than 1500 g).
VUSION should not be used to prevent diaper dermatitis.
The safety of VUSION when used for longer than 7 days is not known. Do not use more than 7 days.
11. Vusion Description
VUSION contains the synthetic antifungal agent, miconazole nitrate (0.25%) USP, zinc oxide (15%) USP, and white petrolatum (81.35%) USP.
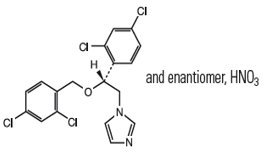
The chemical name of miconazole nitrate is 1-[2, 4-dichloro-ß-{(2,4-dichlorobenzyl)oxy} phenethyl] imidazole mononitrate with empirical formula C18H14Cl4N2O•HNO3 and molecular weight of 479.15. The structural formula of miconazole nitrate is as follows:
The zinc oxide has an empirical formula of ZnO and a molecular weight of 81.39.
The white petrolatum, which is obtained from petroleum and is wholly or nearly decolorized, is a purified mixture of semisolid saturated hydrocarbons having the general chemical formula CnH2n+2. The hydrocarbons consist mainly of branched and unbranched chains. White petrolatum contains butylated hydroxytoluene (BHT) as stabilizer.
Each gram of VUSION contains 2.5 mg of miconazole nitrate USP, 150 mg of zinc oxide USP, and 813.5 mg of white petrolatum USP containing butylated hydroxytoluene, trihydroxystearin, and Chemoderm 1001/B fragrance.
VUSION is a smooth, uniform, white ointment.
12. Vusion - Clinical Pharmacology
12.1 Mechanism of Action
The miconazole component of VUSION is an antifungal agent [see Clinical Pharmacology (12.4)]. The mechanism of action of white petrolatum and zinc oxide for the adjunctive treatment of diaper dermatitis is unknown.
12.2 Pharmacodynamics
The human pharmacodynamics of Vusion is unknown [see Clinical Pharmacology (12.4) for fungal pharmacodynamics].
12.3 Pharmacokinetics
The topical absorption of miconazole from VUSION was studied in immunocompetent male and female infants and children (n=17) with diaper dermatitis complicated by documented candidiasis (microscopic evidence of pseudohyphae and/or budding yeast) ranging in age from 1 month to 21 months. After multiple daily applications to the affected area at every diaper change (approximately 5-12 times per day) for 7 days, the plasma concentrations of miconazole were below the lower limit of quantitation (LOQ) of 0.5 ng/mL in 15 out of 17 (88%) subjects. In the other 2 remaining subjects, the plasma concentrations of miconazole were 0.57 and 0.58 ng/mL, respectively at a single timepoint (4 hours after the last application) on Day 7.
12.4 Microbiology
The miconazole nitrate component in this product has been shown to have in vitro activity against Candida albicans, an organism that is associated with diaper dermatitis. The activity of miconazole nitrate against C. albicans is based on the inhibition of the ergosterol biosynthesis in the cell membrane. The accumulation of ergosterol precursors and toxic peroxides results in cytolysis of the cell. In vitro minimal inhibitory concentration (MIC) test results for C. albicans isolates obtained from treatment failures in Clinical Study 1 [see Clinical Studies (14)] does not appear to indicate that resistance to miconazole nitrate was the reason for treatment failure. The clinical significance of the in vitro activity of miconazole nitrate against C. albicans in the setting of diaper dermatitis is unclear.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of VUSION in animals has not been evaluated.
Miconazole nitrate was negative in a bacterial reverse mutation test, a chromosome aberration test in mice, and micronucleus assays in mice and rats.
Miconazole nitrate had no adverse effect on fertility in a study in rats at oral doses of up to 320 mg/kg/day.
14. Clinical Studies
Study 1 was a double-blind, multicenter study in which VUSION was compared to the zinc oxide and white petrolatum combination treatment and included 236 infants and toddlers with diaper dermatitis, complicated by candidiasis as documented by KOH tests that demonstrated pseudohyphae and/or budding yeasts. Study medication was applied at every diaper change for 7 days.
The primary endpoint was “Overall Cure” and required that subjects be both clinically cured (total resolution of all signs and symptoms of infection) and microbiologically cured (eradication of candidiasis). Primary efficacy was assessed 1 week following the end of treatment, at Day 14.
Study results are shown in the following table.
|
Overall Cure at Day 14 |
||
|
|
VUSION |
Zinc Oxide/White Petrolatum |
|
|
26 (23%) |
12 (10%) |
Two additional studies provided supportive evidence of the clinical efficacy of VUSION in infants and toddlers with diaper dermatitis, some of whom cultured positive for C. albicans. However, candidal infection was not documented in the culture-positive subjects, as microscopic testing (e.g. KOH) was not done. Therefore, the positive culture results may have reflected colonization rather than infection.
16. How is Vusion supplied
16.1 How Supplied
VUSION (miconazole nitrate, zinc oxide and white petrolatum) contains 2.5 mg of miconazole nitrate USP, 150 mg of zinc oxide USP and 813.5 mg of white petrolatum USP per gram. The smooth, uniform, white ointment supplied in an aluminum tube is available as follows:
NDC 0378-9730-50
carton containing one 50 gram tube
17. Patient Counseling Information
See FDA-Approved Patient Labeling
Patients using VUSION should be informed about the following information:
- •
- VUSION should be used only as directed by the health care provider.
- •
- VUSION should not be used as a substitute for frequent diaper changes.
- •
- VUSION should not be used to prevent diaper dermatitis.
- •
- VUSION is for external use only. It is not for oral, ophthalmic, or intravaginal use.
- •
- Gently cleanse the diaper area with lukewarm water or a very mild soap and pat the area dry with a soft towel before applying VUSION.
- •
- Gently apply VUSION to the diaper area with the fingertips after each diaper change. Do not rub VUSION into the skin as this may cause additional irritation.
- •
- Thoroughly wash hands after applying VUSION.
- •
- Treatment should be continued for 7 days, even if there is improvement. Do not use VUSION for longer than 7 days. If symptoms have not improved by day 7, see your health care provider.
- •
- VUSION should not be used on children for whom it is not prescribed.
Patient Information
|
VUSION® (Vu-sion)
|
|
Important Information:VUSION is for use on the skin only. Do not use VUSION in the eyes, mouth, or vagina. |
|
What is VUSION?
It is not known if VUSION is safe and effective for use in incontinent adults. It is not known if VUSION is safe and effective for use in children less than 4 weeks of age or very low birth weight (less than 1500 grams). |
|
Before using VUSION, tell your child’s healthcare provider about all their medical conditions..
|
|
How should I use VUSION?
|
|
What should I avoid while using VUSION?
|
|
What are the possible side effects of VUSION? VUSION may cause serious side effects, including:
These are not all the possible side effects of VUSION. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store VUSION?
|
|
General information about the safe and effective use of VUSION.
|
|
What are the ingredients in VUSION?
|
This Patient Information leaflet has been approved by the U.S. Food and Drug Administration. Revised: 8/2018
VUSION is a registered trademark of Stiefel Laboratories, Inc., a GSK Company, exclusively licensed to the Mylan Companies.
©2018 Mylan Pharmaceuticals Inc. All rights reserved.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Manufactured by:
Confab Laboratories, Inc.
Saint-Hubert, QC, Canada
Revised: 8/2018
CON:MZPOIN:R1
PRINCIPAL DISPLAY PANEL – 50 grams
NDC 0378-9730-50 Rx only
Vusion®
(miconazole nitrate
USP, 0.25%; zinc
oxide USP, 15%;
white petrolatum
USP, 81.35%)
Ointment
For Topical Use Only
50 grams
Usual Dosage: See package insert.
Caution: Not for oral, ophthalmic, or
intravaginal use. Keep out of reach of
children. If seal is damaged or
punctured, do not use, and return
product to place of purchase.
Description: Each gram of VUSION®
Ointment contains 2.5 mg miconazole
nitrate USP, 150 mg zinc oxide USP, and
813.5 mg white petrolatum USP containing
butylated hydroxytoluene, trihyforxystearin,
and Chemoderm 1001/B fragrance.
Store at 20º to 25ºC (68º to 77ºF).
[See USP Controlled Room Temperature.]
See flap for lot number and expiration date.
For additional information, call Mylan at
1-877-446-3679 (1-877-4-INFO-RX).
Serious side effects associated with the use
of this product may be reported to this
number.
VUSION is a registered trademark of
Stiefel Laboratories, Inc., a GSK Company,
exclusively licensed to the Mylan Companies.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in Canada
Mylan.com
CON:9730:50:1C:R2
| VUSION
miconazole nitrate, zinc oxide, white petrolatum ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Pharmaceuticals Inc. (059295980) |
More about Vusion (miconazole / zinc oxide topical)
- Check interactions
- Compare alternatives
- Reviews (4)
- Side effects
- Dosage information
- FDA approval history
- Drug class: topical antifungals