Otiprio Ear Drops: Package Insert / Prescribing Info
Package insert / product label
Generic name: ciprofloxacin
Dosage form: otic suspension
Drug class: Otic anti-infectives
Medically reviewed by Drugs.com. Last updated on Nov 25, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
OTIPRIO® (ciprofloxacin otic suspension), for intratympanic or otic use
Initial U.S. Approval: 1987
Indications and Usage for Otiprio Ear Drops
OTIPRIO is a fluoroquinolone antibacterial indicated for the following conditions:
Otiprio Ear Drops Dosage and Administration
- OTIPRIO is for intratympanic or otic administration by a healthcare professional only. (2.1)
- OTIPRIO is intended for single-patient use with up to two doses available in each vial. (2.1)
- For bilateral otitis media with effusion, administer OTIPRIO as a single intratympanic administration of one 0.1 mL (6 mg) dose into each affected ear, following suctioning of the middle ear effusion. (2.1)
- For acute otitis externa, administer OTIPRIO as a single 0.2 mL (12 mg) administration to the affected ear(s). (2.1)
- See Full Prescribing Information for directions for OTIPRIO dose preparation. (2.2)
Dosage Forms and Strengths
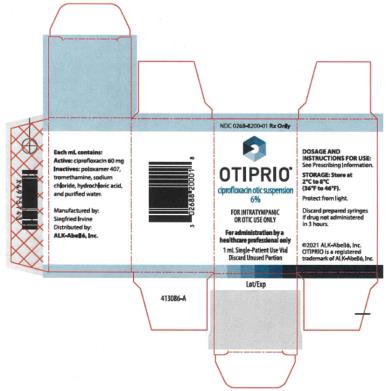
Otic Suspension: Each OTIPRIO vial contains 1 mL of 6% (60 mg/mL) ciprofloxacin otic suspension. (3)
Contraindications
OTIPRIO is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to quinolones, or to any component of OTIPRIO. (4)
Warnings and Precautions
Potential for Microbial Overgrowth: OTIPRIO may result in overgrowth of non-susceptible bacteria and fungi. (5.1)
Adverse Reactions/Side Effects
Otitis Media with Effusion: The most frequently occurring adverse reactions (with an incidence rate greater than 3%) were nasopharyngitis and irritability. (6.1)
Acute Otitis Externa: The most frequently occurring adverse reactions (with an incidence rate of at least 2%) were: ear pruritus, headache, otitis media and ear discomfort. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact ALK-Abelló, Inc. at 1-855-216-6497 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2021
Full Prescribing Information
1. Indications and Usage for Otiprio Ear Drops
OTIPRIO is indicated for
● The treatment of pediatric patients (6 months of age and older) with bilateral otitis media with effusion undergoing tympanostomy tube placement.
● The treatment of acute otitis externa in patients 6 months of age and older due to Pseudomonas aeruginosa and Staphylococcus aureus.
2. Otiprio Ear Drops Dosage and Administration
2.1 Dosage and Important Administration Instructions
● OTIPRIO is for intratympanic or otic administration by a healthcare professional only.
● OTIPRIO is intended for single-patient use, discard unused portion.
● For bilateral otitis media with effusion, administer OTIPRIO as a single intratympanic administration of one 0.1 mL (6 mg) dose into each affected ear of pediatric patients (6 months of age and older), following suctioning of middle ear effusion.
● For acute otitis externa, administer OTIPRIO as a single 0.2 mL (12 mg) administration to the external ear canal of each affected ear of patients aged 6 months and older.
3. Dosage Forms and Strengths
Otic Suspension: Each 1 mL of OTIPRIO contains a white, preservative-free, sterile otic suspension consisting of 6% (60 mg/mL) ciprofloxacin in a single-patient use glass vial.
4. Contraindications
OTIPRIO is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components of OTIPRIO.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Otitis Media with Effusion
In two randomized, sham-controlled Phase 3 clinical trials, 530 pediatric patients with bilateral otitis media with effusion undergoing tympanostomy tube placement were treated with OTIPRIO or sham administered intratympanically as a single dose (0.1 mL to each ear). The median age of the pediatric patients enrolled in the clinical trials was 1.5 years; 62% of patients were 6 months through 2 years of age and 38% of patients were greater than 2 years of age.
Adverse reactions that occurred in at least 3% of OTIPRIO patients and at an incidence greater than sham are presented in Table 1.
| Adverse Reactions | OTIPRIO
(N=357) | Sham
(N=173) |
| Nasopharyngitis | 5% | 4% |
| Irritability | 5% | 3% |
| Rhinorrhea | 3% | 2% |
Acute Otitis Externa
In a single randomized, sham controlled Phase 3 clinical trial, 259 pediatric and adult patients with acute otitis externa were treated with OTIPRIO or sham administered by a healthcare professional to the external ear canal as a single dose (0.2
mL to each affected ear). The median age of the patients enrolled in the clinical trial was 34 years; 26% were pediatric patients (age 3 to 17 years), 65% were adults (age 18 to 64 years), and 8% were elderly patients (age 65 years and older).
Adverse reactions that occurred in at least 2% of OTIPRIO patients and at an incidence greater than sham are presented in Table 2.
| Adverse Reactions | OTIPRIO
(N=127) | Sham
(N=132) |
| Ear Pruritus | 2% | 2% |
| Headache | 2% | 1% |
| Otitis Media | 2% | 1% |
| Ear Discomfort | 2% | 0% |
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Animal reproduction studies have not been conducted with OTIPRIO. No adequate and well-controlled studies have been performed in pregnant women. Because of the negligible systemic exposure associated with clinical administration of OTIPRIO, this product is expected to be of minimal risk for maternal and fetal toxicity when administered to pregnant women.
8.2 Lactation
Risk Summary
Ciprofloxacin is excreted in human milk with systemic administration. However, because of the negligible systemic exposure after otic application, nursing infants of mothers receiving OTIPRIO should not be affected.
8.4 Pediatric Use
The safety and effectiveness of OTIPRIO for the treatment of pediatric patients (6 months of age and older) with bilateral otitis media with effusion undergoing tympanostomy tube placement was established in 530 patients who participated in the Phase 3 trials. The median age of patients enrolled in the clinical trials was 1.5 years; 62% of patients were 6 months through 2 years of age and 38% of patients were greater than 2 years of age [see Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of OTIPRIO for the treatment of acute otitis externa was established in 67 pediatric patients (3 through 17 years of age) who participated in the Phase 3 trial; 57% of patients were 3 through 11 years of age and 43% of patients were 12 through 17 years of age. The safety and efficacy observed in the pediatric patients was no different from the older population. OTIPRIO is indicated for the treatment of acute otitis externa in pediatric patients 6 months of age and older. [see Indications and Usage (1), Dosage and Administration (2), Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of OTIPRIO in infants below 6 months of age have not been established for the treatment of pediatric patients with bilateral otitis media with effusion undergoing tympanostomy tube placement and acute otitis externa.
8.5 Geriatric Use
Clinical studies of OTIPRIO did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients [see Adverse Reactions (6.1) and Clinical Studies (14)].
11. Otiprio Ear Drops Description
OTIPRIO (ciprofloxacin otic suspension) 6% contains the synthetic fluoroquinolone antibacterial, ciprofloxacin. OTIPRIO is for intratympanic administration for otitis media with effusion and otic administration to the external ear canal for acute otitis externa. OTIPRIO is supplied as a white, preservative-free, sterile otic suspension of 6% (w/v) ciprofloxacin in a neutral pH, buffered, isotonic solution in a single-patient use glass vial containing 1 mL. The glass vial is fitted with a stopper not made with natural rubber latex. The inactive ingredients are poloxamer 407, sodium chloride, tromethamine, hydrochloric acid and water for injection (WFI).
The thermosensitive suspension exists as a liquid at room temperature or below and gels when warmed [see How Supplied/Storage and Handling (16)].
Ciprofloxacin has the following nomenclature:
1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.3.
Its chemical structure is as follows:
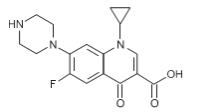
Figure 2: Structure of Ciprofloxacin
12. Otiprio Ear Drops - Clinical Pharmacology
12.1 Mechanism of Action
Ciprofloxacin is a fluoroquinolone antibacterial [see Microbiology (12.4)].
12.3 Pharmacokinetics
The plasma concentration of ciprofloxacin following otic administration of OTIPRIO was not measured.
12.4 Microbiology
Mechanism of Action
The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase, which is needed for the synthesis of bacterial DNA.
Resistance
Bacterial resistance to fluoroquinolones can develop through chromosomally- or plasmid-mediated mechanisms. In vitro studies demonstrated cross-resistance between ciprofloxacin and some fluoroquinolones. There is generally no cross- resistance between ciprofloxacin and other classes of antibacterial agents, such as beta-lactams or aminoglycosides.
Antimicrobial Activity
Ciprofloxacin has been shown to be active against most isolates of the following bacteria:
Gram-positive Bacteria
Staphylococcus aureus
Streptococcus pneumoniae
Gram-negative Bacteria
Haemophilus influenzae
Moraxella catarrhalis
Pseudomonas aeruginosa
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Eight in vitro mutagenicity tests have been conducted with ciprofloxacin, and the test results are listed below:
Salmonella/Microsome Test (Negative)
Escherichia coli DNA Repair Assay (Negative)
Mouse Lymphoma Cell Forward Mutation Assay (Positive)
Chinese Hamster V79 Cell HGPRT Test (Negative)
Syrian Hamster Embryo Cell Transformation Assay (Negative)
Saccharomyces cerevisiae Point Mutation Assay (Negative)
Saccharomyces cerevisiae Mitotic Crossover and Gene Conversion Assay (Negative)
Rat Hepatocyte DNA Repair Assay (Positive)
Thus, 2 of the 8 in vitro tests were positive, but results of the following 3 in vivo test systems gave negative results:
Rat Hepatocyte DNA Repair Assay
Micronucleus Test (Mice)
Dominant Lethal Test (Mice)
Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of 750 mg/kg in mice and 250 mg/kg in rats (for mice and rats respectively, approximately 300 and 200 times the maximum recommended clinical dose of ototopical ciprofloxacin based upon body surface area, assuming total absorption of ciprofloxacin from the ear of a patient treated with OTIPRIO) were administered for up to 2 years, there was no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species.
Fertility studies performed in rats at oral doses of ciprofloxacin up to 100 mg/kg/ day revealed no evidence of impairment. This would be approximately 80 times the maximum recommended clinical dose of ototopical ciprofloxacin based upon body surface area, assuming total absorption of ciprofloxacin from the ear of a patient treated with OTIPRIO.
14. Clinical Studies
14.1 Bilateral Otitis Media with Effusion
Two randomized, multicenter, sham-controlled clinical trials in 532 pediatric patients with bilateral otitis media with effusion undergoing myringotomy with tympanostomy tube placement evaluated the safety and efficacy of OTIPRIO when administered intratympanically as a single dose (NCT 01949155 and NCT 01949142). The median age of patients enrolled in the clinical trials was 1.5 years; 62% of patients were 6 months through 2 years of age and 38% of patients were greater than 2 years of age. The efficacy endpoint for both trials was the cumulative proportion of study treatment failures through Day 15, defined as the occurrence of any of the following events: otorrhea as determined by a blinded assessor on or after 3 days post-surgery, otic or systemic antibacterial drug use for any reason any time post-surgery, as well as patients who missed visits or were lost-to-follow-up. Table 3 presents the results from each Phase 3 trial.
| Trial 1 (N=266) | Trial 2 (N=266) | |||||
|
OTIPRIO |
Sham | Difference (Sham – OTIPRIO) (95% CI) |
OTIPRIO |
Sham | Difference (Sham – OTIPRIO) (95% CI) | |
| Treatment Failure | 25% (44/179) | 45% (39/87) | 20% (8%, 32%)2 | 21% (38/178) | 45% (40/88) | 24% (12%, 36%)2 |
| Reason for Failure1 | ||||||
| Otorrhea | 7% | 11% | 7% | 27% | ||
| Otic antibacterial drugs | 6% | 17% | 5% | 8% | ||
| Systemic antibacterial drugs | 2% | 5% | 3% | 3% | ||
| Lost-to-follow-up and missed visit | 10% | 11% | 6% | 7% | ||
- the earliest occurring treatment failure event, and patients were classified as a treatment failure due only to that component for the remainder of the study
- P-value <0.001 for Cochran-Mantel-Haenszel test (adjusted for age-group)
Administration of OTIPRIO did not lead to impairment in hearing function, middle ear function or tube patency by Day 29.
14.2 Acute Otitis Externa
One randomized multicenter, sham-controlled clinical trial in 262 pediatric and adult patients with unilateral or bilateral acute otitis externa was designed to evaluate the safety and efficacy of OTIPRIO when administered by a healthcare professional as a single dose to the external ear canal to patients aged 6 months and older (NCT 02801370). The median age of patients enrolled in the clinical trials was 34 years; 26% of patients were 3 to 17 years of age, 65% of patients were 18 to 64 years of age, and 8% of patients were greater than 65 years of age. No patients less than 3 years of age were enrolled.
The primary efficacy endpoint was the proportion of patients with clinical response at Day 8. Clinical response was defined as the complete absence of signs and symptoms of acute otitis externa (i.e., tenderness, erythema, edema, and otorrhea as determined by the blinded assessor), and no concomitant systemic or topical antibacterial drug (given in the study ear) was taken for any reason at or prior to the study visit. Table 4 contains the proportions of patients with clinical response at Day 8 in both the intent to treat (ITT) population which contains all subjects who were randomized and did not have group A streptococci cultured on Day 1 and the microbiological ITT population which contains all ITT subjects who had a positive culture for S. aureus or P. aeruginosa on Day 1.
| Study Population | OTIPRIO | Sham | % Difference (OTIPRIO - Sham) (95% CI) |
| Intention to Treat (ITT) N=260 | 69% 90/130 | 46% 60/130 | 23.11
(10.66, 34.62) |
| Microbiological ITT (Mic-ITT) N=108 | 60% 31/52 | 34% 19/56 | 25.72
(6.57, 43.32) |
1 p<0.001 from a Fisher’s exact test. 2 p=0.012 from a Fisher’s exact test.
ITT population = all patients who were randomized and did not have group A streptococci cultured on Day 1.
Mic-ITT population = all ITT patients who had a positive culture for S. aureus or
P. aeruginosa on Day 1.
16. How is Otiprio Ear Drops supplied
OTIPRIO is a sterile, preservative-free, otic suspension of 6% (60 mg/mL, w/v) ciprofloxacin in a neutral pH buffered, isotonic solution containing poloxamer 407.
Each OTIPRIO carton contains 1 mL of 6% (60 mg/mL, w/v) ciprofloxacin in a 2 mL single-patient use glass vial fitted with a stopper not made with natural rubber latex. (NDC 0268-8200-01)
OTIPRIO should be stored at 2 to 8°C (36 to 46°F) until prior to use to prevent thickening during preparation. Protect from light. Store in the original carton until dose preparation.
17. Patient Counseling Information
Advise patients and their caregiver(s) that there may be drainage from the ear the first few days following ear tube surgery, but if the ear becomes painful, or continuous ear discharge is noted, or the patient develops a fever, advise patients and their caregiver(s) to consult their physician.
For acute otitis externa, advise patients and/or their caregivers that if the ear continues to be painful, swollen or itchy after a week, they should consult their physician.
Distributed by:
ALK-Abelló, Inc.
Port Washington, NY 11050 www.otiprio.com
OTIPRIO® is a registered trademark of ALK-Abelló, Inc.
U.S. Patent Nos: 8,318,817, 9,205,048, 9,220,796, 9,233,068, 9,486,405, and 9,603,796
413083-A
| OTIPRIO
otiprio suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ALK-Abello, Inc. (809998847) |
Frequently asked questions
More about Otiprio (ciprofloxacin otic)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: otic anti-infectives
- Breastfeeding