Flura-Drops: Package Insert / Prescribing Info
Package insert / product label
Generic name: sodium fluoride
Dosage form: oral liquid
Drug class: Minerals and electrolytes
Medically reviewed by Drugs.com. Last updated on Sep 16, 2024.
On This Page
Flura-Drops Description
Kirkman 2.21 mg Sodium Fluoride Liquid, Flura-Drops are dye free. Each 4 drop dose of 2.21 mg (full strength) contains 1.0 mg of the fluoride ion (F-) from 2.21 mg sodium fluoride (NaF).
Each 4 drops for oral administration contains sodium fluoride equivalent to 1.0 mg of the fluoride ion and the following inactive ingredients: Purified Water USP, methylparaben and propylparaben.
Flura-Drops - Clinical Pharmacology
Sodium fluoride acts systemically (before tooth eruption) and topically (post eruption) by increasing tooth resistance to acid dissolution, by promoting remineralization, and by inhibiting the carcinogenic microbial process.
Indications and Usage for Flura-Drops
For once daily, self-administered, systemic use as a dental caries preventive in pediatric patients. It has been established that ingestion of fluoridated drinking water (1 ppm F-) during the period of tooth development results in a significant decrease in the incidence of dental caries. Kirkman 2.21 mg Sodium Fluoride Liquid, Flura-Drops were developed to provide systemic fluoride for use as a supplement in pediatric patients from 6 months to 3 years of age and older living in areas where the drinking water fluoride content does not exceed 0.6 ppm F-.
Contraindications
Kirkman 2.21 mg Sodium Fluoride Liquid, Flura-Drops are contraindicated when the fluoride content of drinking water is 0.3 ppm F- or more and should not be administered to pediatric patients under the age of 6 months.
Do not administer Fluoride Liquid (any strength) to pediatric patients under age 6 months.
Warnings
Prolonged daily ingestion of quantities greater than the recommended amount may result in various degrees of dental fluorosis in pediatric patients under age 6 years, especially if the water fluoridation exceeds 0.6 ppm. Read directions carefully before using.
Keep out of the reach of children.
Precautions
General:
Please refer to CONTRAINDICATIONS, WARNINGS, and OVERDOSAGE sections for overdose concerns. Use in pediatric patients below the age of 6 months is not recommended by current American Dental Association and American Academy of Pediatrics guidelines.
Drug Interactions:
Do not eat or drink dairy products within one hour of fluoride administration. Incompatibility of fluoride with dairy foods has been reported due to formation of calcium fluoride which is poorly absorbed.
Nursing Mothers:
It is not known if fluoride ion is excreted in human milk. However, many drugs are excreted in human milk and caution should be exercised when Kirkman 2.21 mg Sodium Fluoride Liquid, Flura-Drops are administered to nursing women.
Adverse Reactions/Side Effects
Allergic rash and other idiosyncrasies have rarely been reported.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Related/similar drugs
Overdosage
Accidental ingestion of large amounts of fluoride may result in acute burning in the mouth and sore tongue. Nausea, vomiting, and diarrhea may occur soon after ingestion (within 30 minutes) and are accompanied by salivation, hematemesis, and epigastric cramping abdominal pain. These symptoms may persist for up to 24 hours. If less than 5 mg sodium fluoride/kg body weight (i.e. less than 2.3 mg sodium fluoride/lb body weight) has been ingested, induce vomiting, give orally soluble calcium (e.g. milk, 5% calcium gluconate, or calcium lactate solution) and immediately seek medical assistance. For accidental ingestion of more than 15 mg sodium fluoride/kg of body weight (i.e. more than 6.9 mg sodium fluoride/lb of body weight), induce vomiting and seek emergency medical help.
Flura-Drops Dosage and Administration
Follow the directions for using this medication provided by your doctor. This medicine may be given undiluted or mixed with a non-dairy liquid.
| Fluoride Content of Drinking Water: | Birth to 6 mos. | 6 mos. to Age 3 | Age 3 to 6 | Age 6 to 16 |
| Less than 0.3 ppm | 0 | 1 drop | 2 drops | 4 drops |
| 0.3 to 0.6 ppm | 0 | 0 | 1 drop | 2 drops |
| Greater than 0.6 ppm | 0 | 0 | 0 | 0 |
Storage and Handling
Store at Controlled Room Temperature, 20–25 °C (68–77 °F). See USP "Controlled Room".
NDC: 58223-517-24
PRINCIPAL DISPLAY PANEL
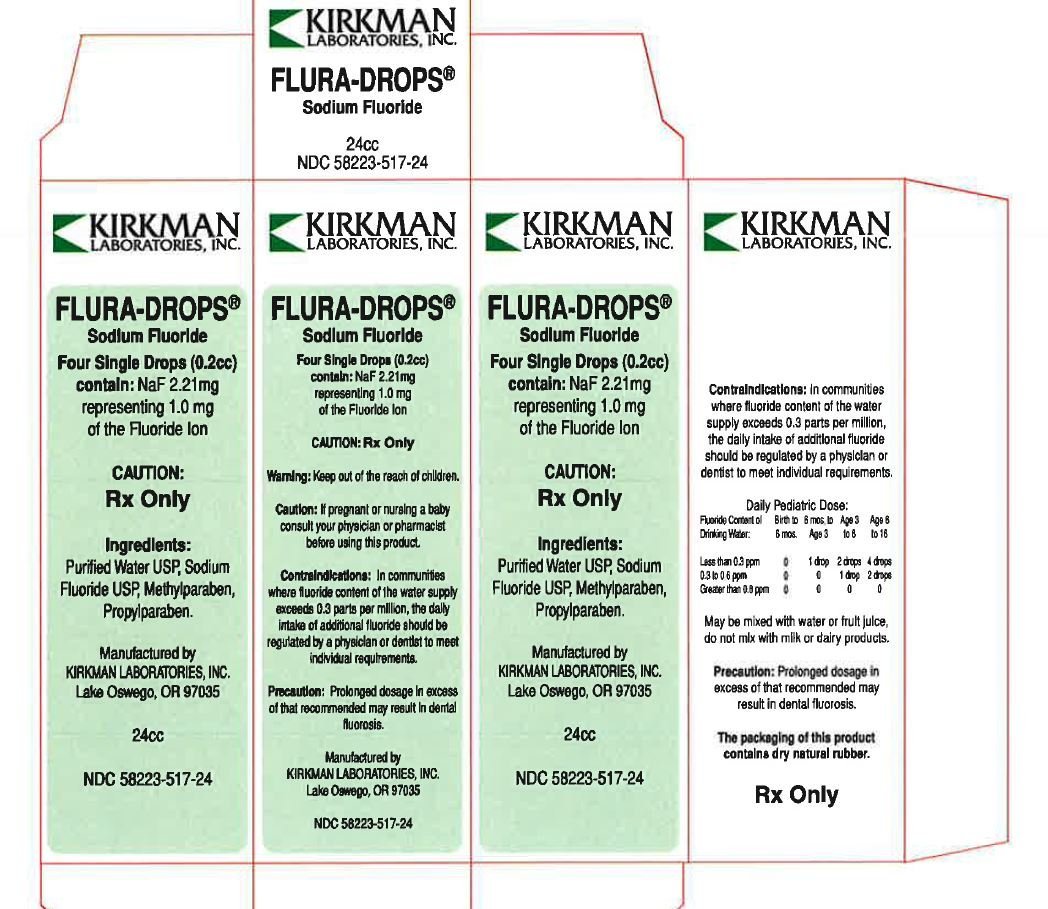
KIRKMAN LABORATORIES, INC.
FLURA-DROPS®
Four Single Drops (0.2cc) contain: NaF 2.21mg representing 1.0 mg of the Fluoride Ion
CAUTION: Rx Only
Ingredients: Purified Water USP, Sodium Fluoride USP, Methylparaben, Propylparaben.
Manufactured by
KIRKMAN LABORATORIES, INC.
Lake Oswego, OR 97035
24cc
NDC 58223-517-24
The packaging of this product contains dry natural rubber.
Rx Only

| FLURA-DROPS
sodium fluoride liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HTO Nevada Inc. (117115846) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KIRKMAN LABORATORIES INC | 180802803 | MANUFACTURE(58223-517) | |
