Crotan Lotion: Package Insert / Prescribing Info
Package insert / product label
Generic name: crotamiton
Dosage form: lotion
Drug class: Topical anti-infectives
Medically reviewed by Drugs.com. Last updated on Apr 23, 2024.
On This Page
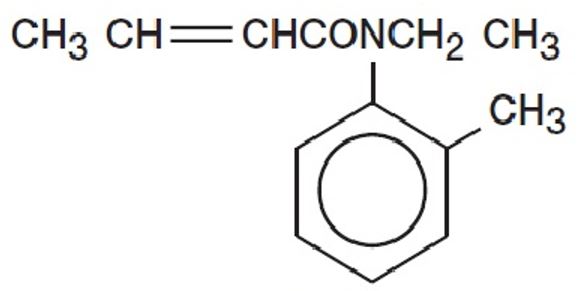
CROTAN ™ (crotamiton USP 10%) is a scabicidal and antipruritic agent as a lotion for topical use only. Crotamiton is a colorless to slightly yellowish oil, having a faint amine-like odor. It is miscible with alcohol and with methanol. Crotamiton is a mixture of the cis and trans isomers. Its molecular weight is 203. 28. Crotamiton is N-ethyl-N(o-methyl-phenyl) 2-butenamide and its structural formula is:
CROTAN lotion contains crotamiton USP 10% (100mg/ml) in a creamy lotion base containing purified water, light mineral oil, propylene glycol, cetearyl alcohol (and) cetearth-20, cetyl alcohol, lanolin, benzyl alcohol, carbomer 971P, sodium hydroxide with citric acid (for pH adjustment).
CLINICAL PHARMACOLOGY: CROTAN ™ lotion has scabicial and antipruritic actions. The mechanisms of these actions are not known. The pharmacokinetics of crotamiton and its degree of systemic absorption following topical application have not been determined.
Geriatric Use: Clinical studies with CROTAN (crotamiton USP) lotion did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
ADVERSE REACTIONS: Primary irritation reactions such as dermatitis, pruritus and rash, and allergic sensitivity reactions have been reported in a few patients.
To report SUSPECTED ADVERSE REACTIONS, contact Marnel Pharmaceuticals at 1-888-850-2905 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE: There is no specific informaton on the effect of overtreatment with repeated topical applications in humans. A death was reported but cause was not confirmed. Accidental oral ingestion may be accompanied by burning sensation in the mouth, irritation of the buccal, esophageal and gastric mucosa, nausea, vomiting, abdominal pain.
If accidental ingestion occurs, call your Poison Control Center.
DOSAGE AND ADMINISTRATION:
SHAKE WELL BEFORE USE.
In Scabies: Thoroughly massage into the skin of the whole body, from the chin down, paying particular attention to all folds and creases. A second application is advisable 24 hours later. Clothing and bed linen should be changed the next morning. A cleansing bath should be taken 48 hours after the last application.
In Pruritis: Massage gently into affected areas until medication is completely absorbed. Repeat as needed.
DIRECTIONS FOR PATIENTS WITH SCABIES:
1. Take a routine bath or shower. Thoroughly massage CROTAN ™ lotion into the skin from the chin to the toes including folds and creases.
2. Put CROTAN lotion under fingernails after trimming the fingernails short, because scabies are likely to remain there. A toothbrush can be used to apply the CROTAN lotion under the fingernails. Immediately after use, the toothbrush should be wrapped in paper and thrown away. Use of the brush in the mouth could lead to poisoning.
3. A second application is advisable 24 hours leater.
4. Clothing and bed linen should be changed the next day. Contaminated clothing and bed linen may be dry-cleaned or washed in the hot cycle of the washing machine.
5. A cleansing bath should be taken 48 hours after the last application.
INDICATIONS AND USAGE: For eradication of scabies ( Sarcoptes scabiei) and for symptomatic treatment of pruritic skin.
CONTRAINDICATIONS: CROTAN lotion should not be applied topically to patients who develop a sensitivity or are allergic to it or who manifest a primary irritation response to topical medications.
WARNINGS: If severe irritation or sensitization develops, treatment with this product should be discontinued and appropriate therapy instituted.
PRECAUTIONS: General: CROTAN lotion should not be applied in the eyes or mouth because it may cause irritation. It should not be applied to acutely inflamed skin or raw or weeping surfaces until acute inflammation has subsided.
Carcinogenesis, Mutangenesis, Impairment of Fertility: Long-term carcinogenicity studies in animals have not been conducted.
Pregnancy (Category C): Animal reproduction studies have not been conducted with CROTAN (crotamiton USP) lotion. It is also not known whether CROTAN can cause fetal harm when applied to a pregnant woman or can affect reproduction capacity. CROTAN should be given to a pregnant woman only if clearly needed.
HOW SUPPLIED: CROTAN (crotamitan USP) lotion, 10% is available in:
60 gm ( NDC: 0682-0051-20)
237 gm ( NDC: 0682-0051-10)
454 gm ( NDC: 0682-0051-30)
Rx ONLY
Mfd For:
Marnel Pharmaceuticals LLC
Charleston, South Carolina USA
888-850-2905
Rev July 2021
| CROTAN
crotamiton lotion |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Marnel Pharmaceuticals, Inc. (080161449) |
| Registrant - Marnel Pharmaceuticals, Inc. (080161449) |
Related/similar drugs
More about Crotan (crotamiton topical)
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical anti-infectives
- En español

