Conray: Package Insert / Prescribing Info
Package insert / product label
Generic name: iothalamate meglumine
Dosage form: injection
Drug class: Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on May 7, 2025.
On This Page
Conray Description
Conray is a sterile aqueous solution intended for use as a diagnostic radiopaque medium. Conray contains 60% w/v iothalamate meglumine, which is 1-deoxy-1-(methylamino)-D-glucitol 5-acetamido-2,4,6 triiodo-N-methylisophthalamate (salt), and has the following structural formula:
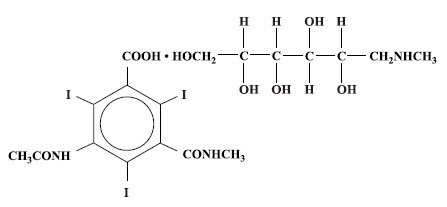
Each milliliter contains 600 mg of iothalamate meglumine, 0.09 mg edetate calcium disodium as a stabilizer and 0.125 mg of monobasic sodium phosphate as a buffer. The solution provides 28.2% (282 mg/mL) organically bound iodine. Conray has an osmolarity of approximately 1000 mOsmol per liter, an osmolality of approximately 1400 mOsmol per kilogram and is, therefore, hypertonic under conditions of use. The viscosity (cps) is approximately 6 at 25°C and 4 at 37°C. The pH is 6.5 to 7.7.
Conray is a clear solution containing no undissolved solids. Crystallization does not occur at normal room temperatures. It is supplied in containers from which the air has been displaced by nitrogen.
Conray - Clinical Pharmacology
Following intravascular injection, Conray is rapidly transported through the circulatory system to the kidneys and is excreted unchanged in the urine by glomerular filtration. The pharmacokinetics of intravascularly administered radiopaque contrast media are usually best described by a two compartment model with a rapid alpha phase for drug distribution and a slower beta phase for drug elimination. In patients with normal renal function, the alpha and beta half-lives of Conray were approximately 10 and 90 minutes, respectively.
Angiography may be performed following intravascular injection which will permit visualization until significant hemodilution occurs.
Renal accumulation is sufficiently rapid that maximum radiographic density in the calyces and pelves occurs, in most instances, about 3 to 8 minutes after injection. In patients with impaired renal function, diagnostic opacification frequently is achieved only after prolonged periods.
Injectable iodinated contrast agents are excreted either through the kidneys or through the liver. These two excretory pathways are not mutually exclusive, but the main route of excretion seems to be related to the affinity of the contrast medium for serum albumin. Iothalamate salts are poorly bound to serum albumin, and are excreted mainly through the kidneys.
The liver and small intestine provide the major alternate route of excretion. In patients with severe renal impairment, the excretion of this contrast medium through the gallbladder and into the small intestine sharply increases.
Iothalamate salts cross the placental barrier in humans and are excreted unchanged in human milk.
The biliary system, pancreatic duct or joint spaces may be visualized by the direct injection of contrast medium into the region to be studied.
CT Scanning of the Head
When used for contrast enhancement in computed tomographic brain scanning, the degree of enhancement is directly related to the amount of iodine administered. Rapid injection of the entire dose yields peak blood iodine concentrations immediately following the injection, which fall rapidly over the next five to ten minutes. This can be accounted for by the dilution in the vascular and extracellular fluid compartments which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached by about ten minutes; thereafter, the fall becomes exponential. Maximum contrast enhancement frequently occurs after peak blood iodine levels are reached. The delay in maximum contrast enhancement can range from five to forty minutes, depending on the peak iodine levels achieved and the cell type of the lesion. This lag suggests that the contrast enhancement of the image is at least in part dependent on the accumulation of iodine within the lesion and outside the blood pool.
In brain scanning, the contrast medium (Conray) does not accumulate in normal brain tissue due to the presence of the “blood brain barrier.” The increase in x-ray absorption in the normal brain is due to the presence of the contrast agent within the blood pool. A break in the blood brain barrier, such as occurs in malignant tumors of the brain, allows accumulation of contrast medium within the interstitial tumor tissue; adjacent normal brain tissue does not contain the contrast medium.
The image enhancement of non-tumoral lesions, such as arteriovenous malformations and aneurysms, is dependent on the iodine content of the circulating blood pool.
When used for cranial computerized angiotomography, rapid bolus injection and/or infusion combined with rapid CT scanning will provide clear delineation of the cerebral vessels.
CT Scanning of the Body1
In non-neural tissues (during CT of the body), Conray diffuses rapidly from the vascular to the extra-vascular space. Increase in x-ray absorption is related to blood flow, concentration of the contrast medium and extraction of the contrast medium by interstitial tissue, since no barrier exists; contrast enhancement is thus due to the relative differences in extra-vascular diffusion between normal and abnormal tissue, a situation quite different than that in the brain.
The pharmacokinetics of Conray in normal and abnormal tissues has been shown to be variable.
Enhancement of CT with Conray may be of benefit in establishing diagnoses of certain lesions in some sites with greater assurance than is possible with unenhanced CT and in supplying additional features of the lesions. In other cases, the contrast medium may allow visualization of lesions not seen with CT alone or may help to define suspicious lesions seen with unenhanced CT.
Contrast enhancement appears to be greatest within the 30 to 90 seconds after bolus administration of the contrast agent, and after intra-arterial, rather than intravenous, administration. Therefore, the use of a continuous scanning technique (a series of 2 to 3 second scans beginning at the injection - dynamic CT scanning) may improve enhancement and diagnostic assessment of tumors and other lesions, such as an abscess, occasionally revealing more extensive disease. A cyst, or similar non-vascularized lesion, may be distinguished from vascularized solid lesions by comparing enhanced and unenhanced scans; non-vascularized lesions show no change in CT number, whereas vascularized lesions would show an increase. The latter might be benign, malignant or normal, but it is unlikely that it would be a cyst, hematoma, or other non-vascularized lesion.
Because unenhanced scanning may provide adequate information in the individual patient, the decision to employ contrast enhancement, which is associated with additional risk and increased exposure, should be based upon a careful evaluation of clinical, other radiological, and unenhanced CT findings.
Indications and Usage for Conray
Conray is indicated for use in excretory urography, cerebral angiography, peripheral arteriography, venography, arthrography, direct cholangiography, endoscopic retrograde cholangiopancreatography, contrast enhancement of computed tomographic brain images, cranial computerized angiotomography, intravenous digital subtraction angiography and arterial digital subtraction angiography.
Conray may also be used for enhancement of computed tomographic scans performed for detection and evaluation of lesions in the liver, pancreas, kidneys, abdominal aorta, mediastinum, abdominal cavity and retroperitoneal space. Continuous or multiple scans separated by intervals of 1 to 3 seconds during the first 30 to 90 seconds post-injection of the contrast medium (dynamic CT scanning) may provide enhancement of diagnostic significance, and may be of benefit in establishing diagnoses of certain lesions in these sites with greater assurance than is possible with CT alone, and in supplying additional features of the lesions. In other cases, the contrast agent may allow visualization of lesions not seen with CT alone, or may help to define suspicious lesions seen with unenhanced CT (see CLINICAL PHARMACOLOGY). Subsets of patients in whom delayed body CT scans might be helpful have not been identified. Inconsistent results have been reported and abnormal and normal tissues may be isodense during the time frame used for delayed CT scanning. The risks of such indiscriminate use of contrast media are well known and such use is not recommended. At present, consistent results have been documented using dynamic CT techniques only.
Contraindications
Refer to PRECAUTIONS, General, concerning hypersensitivity. Conray should not be used for myelography. Arthrography should not be performed if infection is present in or near the joint. Percutaneous transhepatic cholangiography is contraindicated in patients with coagulation defects and prolonged prothrombin times. Endoscopic retrograde cholangiopancreatography is contraindicated during an acute attack of pancreatitis or during severe clinically evident cholangitis and in patients in whom endoscopy is prohibited.
Warnings
SEVERE ADVERSE EVENTS - INADVERTENT INTRATHECAL ADMINISTRATION: Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to ensure that this drug product is not administered intrathecally.
Ionic iodinated contrast media inhibit blood coagulation, in vitro, more than nonionic contrast media. Nonetheless, it is prudent to avoid prolonged contact of blood with syringes containing ionic contrast media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended, including close attention to guidewire and catheter manipulation, use of manifold systems and/or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease, but not eliminate, the likelihood of in vitro clotting.
Serious or fatal reactions have been associated with the administration of iodine-containing radiopaque media. It is of utmost importance to be completely prepared to treat any contrast medium reaction.
Serious neurologic sequelae, including permanent paralysis, have been reported following cerebral arteriography, selective spinal arteriography and arteriography of vessels supplying the spinal cord. The intravascular injection of a contrast medium should never be made following the administration of vasopressors since they strongly potentiate neurologic effects.
In patients with subarachnoid hemorrhage, a rare association between contrast administration and clinical deterioration, including convulsions and death, has been reported. Therefore, administration of intravascular iodinated ionic contrast media in these patients should be undertaken with caution.
A definite risk exists in the use of intravascular contrast agents in patients who are known to have multiple myeloma. In such instances, anuria has developed, resulting in progressive uremia, renal failure and eventually death. Although neither the contrast agent nor dehydration has separately proved to be the cause of anuria in myeloma, it has been speculated that the combination of both may be causative factors. The risk in myelomatous patients is not a contraindication to the procedure; however, partial dehydration in the preparation of these patients for the examination is not recommended, since this may predispose to precipitation of myeloma protein in the renal tubules. No form of therapy, including dialysis, has been successful in reversing the effect. Myeloma, which occurs most commonly in persons over 40, should be considered before instituting intravascular administration of contrast agents.
Administration of radiopaque materials to patients known or suspected to have pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure, and measures for treatment of a hypertensive crisis should be available.
Contrast media have been shown to promote the phenomenon of sickling in individuals who are homozygous for sickle cell disease when the material is injected intravenously or intra-arterially.
Convulsions have occurred in patients with primary or metastatic cerebral lesions following the administration of iodine-containing radiopaque media for the contrast enhancement of CT brain images.
In patients with advanced renal disease, iodinated contrast media should be used with caution, and only when the need for the examination dictates, since excretion of the medium may be impaired. Patients with combined renal and hepatic disease, those with severe hypertension or congestive heart failure, and recent renal transplant recipients may present an additional risk.
Renal failure has been reported in patients with liver dysfunction who were given an oral cholecystographic agent followed by an intravascular iodinated radiopaque agent, and also in patients with occult renal disease, notably diabetics and hypertensives. In these classes of patients, there should be no fluid restriction and every attempt made to maintain normal hydration, prior to contrast medium administration, since dehydration is the single most important factor influencing further renal impairment.
Acute renal failure has been reported in diabetic patients with diabetic nephropathy and in susceptible non-diabetic patients (often elderly with pre-existing renal disease) following the administration of iodinated contrast agents. Therefore, careful consideration of the potential risks should be given before performing this radiographic procedure in these patients.
Caution should be exercised in performing contrast medium studies in patients with endotoxemia and/or those with elevated body temperatures.
Reports of thyroid storm occurring following the intravascular use of iodinated radiopaque agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule, suggest that this additional risk be evaluated in such patients before use of this drug. Iodine containing contrast agents may alter the results of thyroid function tests which depend on iodine estimation, e.g. PBI and radioactive iodine uptake studies. Such tests, if indicated, should be performed prior to the administration of this preparation.
Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age:
Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast media (ICM) in pediatric patients 0 to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at the greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
Severe Cutaneous Adverse Reactions: Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering Conray to patients with a history of a severe cutaneous adverse reaction to Conray.
Precautions
General
Diagnostic procedures which involve the use of iodinated intra-vascular contrast agents should be carried out under the direction of personnel skilled and experienced in the particular procedure to be performed. All procedures utilizing contrast media carry a definite risk of producing adverse reactions. While most reactions may be minor, life threatening and fatal reactions may occur without warning. The risk-benefit factor should always be carefully evaluated before such a procedure is undertaken. A fully equipped emergency cart, or equivalent supplies and equipment, and personnel competent in recognizing and treating adverse reactions of all severity, or situations which may arise as a result of the procedure, should be immediately available at all times. If a serious reaction should occur, immediately discontinue administration. Since severe delayed reactions have been known to occur, emergency facilities and competent personnel should be available for at least 30 to 60 minutes after administration (see ADVERSE REACTIONS).
Preparatory dehydration is dangerous and may contribute to acute renal failure in infants, young children, the elderly, patients with pre-existing renal insufficiency, patients with advanced vascular disease and diabetic patients.
Severe reactions to contrast media often resemble allergic responses. This has prompted the use of several provocative pretesting methods, none of which can be relied on to predict severe reactions. No conclusive relationship between severe reactions and antigen-antibody reactions or other manifestations of allergy has been established. The possibility of an idiosyncratic reaction in patients who have previously received a contrast medium without ill effect should always be considered. Prior to the injection of any contrast medium, the patient should be questioned to obtain a medical history with emphasis on allergy and hypersensitivity. A positive history of bronchial asthma or allergy, including food, a family history of allergy, or a previous reaction or hypersensitivity to a contrast agent, may imply a greater than usual risk. Such a history, by suggesting histamine sensitivity and consequently proneness to reactions, may be more accurate than pre-testing in predicting the potential for reaction, although not necessarily the severity or type of reaction in the individual case. A positive history of this type does not arbitrarily contraindicate the use of a contrast agent, when a diagnostic procedure is thought essential, but does call for caution (see ADVERSE REACTIONS).
Prophylactic therapy including corticosteroids and antihistamines should be considered for patients who present with a strong allergic history, a previous reaction to a contrast medium, or a positive pretest, since the incidence of reaction in these patients is two to three times that of the general population. Adequate doses of corticosteroids should be started early enough prior to contrast medium injection to be effective and should continue through the time of injection and for 24 hours after injection. Antihistamines should be administered within 30 minutes of the contrast medium injection. Recent reports indicate that such pre-treatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity. A separate syringe should be used for these injections.
General anesthesia may be indicated in the performance of some procedures in young or uncooperative children and in selected adult patients; however, a higher incidence of adverse reactions has been reported in these patients. This may be attributable to the inability of the patient to identify untoward symptoms, or to the hypotensive effect of anesthesia, which can prolong the circulation time and increase the duration of contact of the contrast agent.
Angiography should be avoided whenever possible in patients with homocystinuria because of the risk of inducing thrombosis and embolism.
Information for Patients
Patients receiving iodinated intravascular contrast agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease or known thyroid disease (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food or if you had any reactions to previous injections of dyes used for x-ray procedures (see PRECAUTIONS, General).
- Inform your physician about any other medications you are currently taking, including non-prescription drugs.
- Consult with your physician if, at some future date, any thyroid tests are planned. The iodine in this agent may interfere with later thyroid tests.
- Advise patients to inform their physician if they develop a rash after receiving Conray.
- Advise parents/caregivers about the risk of developing thyroid dysfunction after Conray administration. Advise parents/caregivers about when to seek medical care for their child to monitor for thyroid dysfunction (see WARNINGS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential. However, animal studies suggest that this drug is not mutagenic and does not affect fertility in males or females.
Pregnancy Category B
Reproduction studies have been performed in mice, rats, and rabbits at doses up to 6.6 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Conray. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Iothalamate salts are excreted unchanged in human milk. Because of the potential for adverse effects in nursing infants, bottle feedings should be substituted for breast feedings for 24 hours following the administration of this drug.
(Precautions for specific procedures receive comment under that procedure.)
Pediatric Use
Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates; some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 to 3 years of age based on underlying risk factors, especially in term and preterm neonates (see WARNINGS and ADVERSE REACTIONS).
Adverse Reactions/Side Effects
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physio-chemical properties of the contrast media, the dose and speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, the mode of injection and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
Fatalities have been reported following the administration of iodine-containing contrast agents. Based upon clinical literature, the incidence of death is reported to range from one in 10,000 (0.01 percent) to less than one in 100,000 (0.001 percent).
The following adverse reactions have been observed in conjunction with the use of iodine-containing contrast agents.
The most frequent adverse reactions are nausea, vomiting, facial flush and a feeling of body warmth. These are usually of brief duration. Other reactions include the following:
Hypersensitivity reactions: Dermal manifestations of urticaria with or without pruritus, erythema and maculopapular rash. Dry mouth. Sweating. Conjunctival symptoms. Facial, peripheral and angioneurotic edema. Symptoms related to the respiratory system include sneezing, nasal stuffiness, coughing, choking, dyspnea, chest tightness and wheezing, which may be initial manifestations of more severe and infrequent reactions including asthmatic attack, laryngospasm and bronchospasm with or without edema, pulmonary edema, apnea and cyanosis. Rarely, these allergic-type reactions can progress into anaphylaxis with loss of consciousness and coma and severe cardiovascular disturbances.
Cardiovascular reactions: Generalized vasodilation, flushing and venospasm. Occasionally, thrombosis or rarely, thrombophlebitis. Red blood cell clumping and agglutination, crenation and interference in clot formation. Extremely rare cases of disseminated intravascular coagulation resulting in death have been reported. Severe cardiovascular responses include rare cases of hypotensive shock, coronary insufficiency, cardiac arrhythmia, fibrillation and arrest. These severe reactions are usually reversible with prompt and appropriate management; however, fatalities have occurred.
Endocrine reactions: Hyperthyroidism, hypothyroidism.
Skin and Subcutaneous Tissue Disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)].
Technique reactions: Extravasation with burning pain, hematomas, ecchymosis and tissue necrosis, paresthesia or numbness, vascular constriction due to injection rate, thrombosis and thrombophlebitis.
Neurological reactions: Spasm, convulsions, aphasia, syncope, paresis, paralysis resulting from spinal cord injury and pathology associated with syndrome of transverse myelitis, visual field losses which are usually transient but may be permanent, coma and death.
Other reactions: Headache, trembling, shaking, chills without fever and lightheadedness. Temporary renal shutdown or other nephropathy.
(Adverse reactions to specific procedures receive comment under that procedure.)
Related/similar drugs
Overdosage
Overdosage may occur. The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular system. The symptoms may include cyanosis, bradycardia, acidosis, pulmonary hemorrhage, convulsions, coma and cardiac arrest. Treatment of an overdose is directed toward the support of all vital functions and prompt institution of symptomatic therapy.
Iothalamate salts are dialyzable.
The intravenous LD50 value of various concentrations of Iothalamate Meglumine (in grams of iodine/kilogram body weight) varied from 5.7 to 8.9 g/kg in mice and 9.8 to 11.2 g/kg in rats. The LD50 values decrease as the rate of injection increases.
Conray Dosage and Administration
It is advisable that Conray be at or close to body temperature when injected.
The patient should be instructed to omit the meal that precedes the examination. Appropriate premedication, which may include a barbiturate, tranquilizer or analgesic drug, may be administered prior to the examination.
A preliminary film is recommended to check the position of the patient and the x-ray exposure factors.
If a minor reaction occurs during administration, the injection should be slowed or stopped until the reaction has subsided. If a major reaction occurs, the injection should be discontinued immediately.
Under no circumstances should either corticosteroids or antihistamines be mixed in the same syringe with the contrast medium because of a potential for chemical incompatibility.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
EXCRETORY UROGRAPHY
Following intravenous injection, Conray is rapidly excreted by the kidneys. Conray may be visualized in the renal parenchyma 30 seconds following bolus injection. Maximum radiographic density in the calyces and pelves occurs in most instances within 3 to 8 minutes after injection. In patients with severe renal impairment contrast visualization may be substantially delayed.
Patient Preparation
Appropriate preparation of the patient is important for optimal visualization. A low residue diet is recommended for the day preceding the examination and a laxative is given the evening before the examination, unless contraindicated.
Precautions
Infants and small children should not have any fluid restrictions prior to excretory urography. Injections of Conray represent an osmotic load which, if superimposed on increased serum osmolality due to partial dehydration, may magnify hypertonic dehydration (see WARNINGS and PRECAUTIONS, General concerning preparatory dehydration).
Usual Dosage
Adults - The usual dose is 30 to 60 mL. Children 14 years of age and over, of average weight, may receive the adult dose. The total dose is normally injected within 30 to 90 seconds. Higher dosage may be indicated to achieve optimum results in instances where poor visualization may be anticipated (e.g., elderly patients or patients with impaired renal function). When nephrograms and/or sequential urograms are desired, the total dose should be rapidly injected, normally within 15 to 30 seconds.
The dosage for children is reduced in proportion to age and body weight. The following approximate schedule is recommended for infants and children, based on a dosage of about 0.5 mL/kg of body weight:
| Under 6 months of age | 5 mL |
| 6-12 months | 8 mL |
| 1-2 years | 10 mL |
| 2-5 years | 12 mL |
| 5-8 years | 15 mL |
| 8-12 years | 18 mL |
| 12-14 years | 20-30 mL |
CEREBRAL ANGIOGRAPHY
Conray may be used to visualize the cerebral vasculature by any of the accepted techniques.
Patient Preparation
Cerebral angiography is normally performed with local or general anesthesia (see PRECAUTIONS, General). Premedication may be employed as indicated.
A preliminary radiograph is usually made prior to injection of the contrast agent.
Precautions
In addition to the general precautions previously described, cerebral angiography should be performed with special caution in patients with advanced arteriosclerosis, severe hypertension, cardiac decompensation, senility, recent cerebral thrombosis or embolism, and migraine.
Adverse Reactions
The major sources of cerebral arteriographic adverse reactions appear to be related to repeated injections of the contrast material, administration of doses higher than those recommended, the presence of occlusive atherosclerotic vascular disease and the method and technique of injection.
Adverse reactions are normally mild and transient. A feeling of warmth in the face and neck is frequently experienced. Infrequently, a more severe burning discomfort is observed.
Serious neurological reactions that have been associated with cerebral angiography and not listed under the general Adverse Reactions include stroke, amnesia and respiratory difficulties.
Cardiovascular reactions that may occur with some frequency are bradycardia and decrease in systemic blood pressure. The blood pressure change is transient and usually requires no treatment.
Usual Dosage
The usual dosage employed varies with the site and method of injection and the age, condition and weight of the patient. In adults, carotid and vertebral angiography, by either the percutaneous needle or catheter methods, is usually performed with a single rapid injection of 6 to 10 mL. Additional injections are made as indicated. Retrograde brachial cerebral angiography, in adults, is usually performed with a single rapid injection of 35 to 50 mL into the right brachial artery. Other dosages may be employed depending upon the vessel injected and the procedure followed. The dose for children is reduced in approximate proportion to age and body weight.
PERIPHERAL ARTERIOGRAPHY AND VENOGRAPHY
Conray may be injected to visualize the arterial and venous peripheral circulation. Arteriograms of the upper and lower extremities may be obtained by any of the established techniques. Most frequently, a percutaneous injection is made into the brachial artery in the arm or the femoral artery in the leg. Venograms are obtained by injection into an appropriate vein in the upper and lower extremity.
Patient Preparation
The procedure is normally performed with local or general anesthesia (see PRECAUTIONS, General). Premedication may be employed as indicated.
A preliminary radiograph is usually made prior to the injection of the contrast agent.
Precautions
In addition to the general precautions previously described, moderate decreases in blood pressure occur frequently with intra-arterial (brachial) injections. This change is usually transient and requires no treatment; however, the blood pressure should be monitored for approximately ten minutes following injection. Special care is required when venography is performed in patients with suspected thrombosis, phlebitis, severe ischemic disease, local infection or a totally obstructed venous system. In the presence of venous stasis, vein irrigation with normal saline should be considered following the procedure. Venography is optimally performed with a more dilute solution such as Conray 43 (Iothalamate Meglumine Injection USP 43%).
Extreme caution during injection of the contrast agent is necessary to avoid extravasation and fluoroscopy is recommended. This is especially important in patients with severe arterial or venous disease.
Adverse Reactions
In addition to the general adverse reactions previously described, hemorrhage and thrombosis have occurred at the puncture site of the percutaneous injection. Brachial plexus injury has been reported following axillary artery injection. Thrombophlebitis, syncope and very rare cases of gangrene have been reported following venography.
Usual Dosage
Peripheral Arteriography: In adults a single rapid injection of 20 to 40 mL is normally sufficient to visualize the entire extremity. The dose for children is reduced in proportion to body weight. Venography: The usual dose for adults is a single rapid injection of 20 to 40 mL. The dose for children is reduced in proportion to body weight. Following the procedure, the venous system should be flushed with either 5% dextrose in water (D5W) or normal saline (Sodium Chloride Injection USP) or the contrast medium should be removed by leg massage and/or leg elevation.
ARTHROGRAPHY
Precautions
In addition to the general precautions previously described, strict aseptic technique is required to prevent the introduction of infection. Fluoroscopic control should be used to ensure proper introduction of the needle into the synovial space and prevent extracapsular injection. Aspiration of excessive synovial fluid will reduce the pain on injection and prevent the rapid dilution of the contrast agent. It is important that undue pressure not be exerted during the injection.
Adverse Reactions
In addition to the general adverse reactions previously described arthrography may induce joint pain or discomfort which is usually mild and transient but occasionally may be severe and persist for 24 to 48 hours following the procedure. Effusion requiring aspiration may occur in patients with rheumatoid arthritis.
Usual Dosage
Arthrography is usually performed under local anesthesia. The amount of contrast agent required is solely dependent on the size of the joint to be injected and the technique employed.
The following dosage schedule for normal adult joints should serve only as a guide since joints may require more or less contrast medium for optimal visualization. Dosage should be reduced for children in proportion to body weight.
| Knee, hip | 5-15 mL |
| Shoulder, ankle | 5-10 mL |
| Other | 1-4 mL |
Passive or active manipulation is used to disperse the medium throughout the joint space.
The lower volumes of contrast medium are usually employed for double contrast examinations. Following the injection of the contrast medium 50 to 100 cc of either filtered room air or carbon dioxide is introduced for examination of the knee and lesser volumes for other joints. The concomitant use of epinephrine 1:1000 will reduce the rate of contrast medium absorption as well as the production of synovial fluids and consequent dilution of the medium.
DIRECT CHOLANGIOGRAPHY
Precautions
In addition to the general precautions previously described, in the presence of acute pancreatitis, direct cholangiography, if necessary, should be employed with caution, injecting no more than 5 to 10 mL without undue pressure. Percutaneous transhepatic cholangiography should only be attempted when compatible blood for potential transfusions is in readiness and emergency surgical facilities are available. The patient should be carefully monitored for at least 24 hours to ensure prompt detection of bile leakage and hemorrhage. Appropriate premedication of the patient is recommended and drugs which are cholespastic, such as morphine, should be avoided. Respiratory movements should be controlled during introduction of the needle.
Adverse Reactions
Adverse reactions may often be attributed to injection pressure or excessive volume of the medium resulting in overdistention of the ducts and producing local pain.
Some of the medium may enter the pancreatic duct which may result in pancreatic irritation. Occasionally, nausea, vomiting, fever, and tachycardia have been observed. Pancholangitis resulting in liver abscess or septicemia has been reported.
In percutaneous transhepatic cholangiography, some discomfort is common, but severe pain is unusual. Complications of the procedure are often serious and have been reported in 4 to 6 percent of patients. These reactions have included bile leakage and biliary peritonitis, gall bladder perforation, internal bleeding (sometimes massive), blood-bile fistula resulting in septicemia involving gram-negative organisms, and tension pneumothorax from inadvertent puncture of the diaphragm or lung. Bile leakage is more likely to occur in patients with obstructions that cause unrelieved high biliary pressure.
Dosage and Administration
It is advisable that Conray be at or close to body temperature when injected. The injection is made slowly without undue pressure, taking the necessary precautions to avoid the introduction of bubbles.
Operative – The usual dose is 10 mL but as much as 25 mL may be needed depending upon the caliber of the ducts. If desired, the contrast agent may be diluted 1:1 with Sodium Chloride Injection USP using strict aseptic procedures. Following surgical exploration of the ductal system, repeat studies may be performed before closure of the abdomen, using the same dose as before.
Postoperative – Postoperatively, the ductal system may be examined by injection of the contrast agent through an in-place T-tube. These delayed cholangiograms are usually made from the fifth to the tenth postoperative day prior to removal of the T-tube. The usual dose is the same as for operative cholangiography.
Percutaneous Transhepatic Cholangiography – This procedure is recommended for carefully selected patients for the differential diagnosis of jaundice due to extrahepatic biliary obstruction or parenchymal disease. The procedure is only employed where oral or intravenous cholangiography and other procedures have failed to provide the necessary information. In obstructed cases, percutaneous transhepatic cholangiography is used to determine the cause and site of obstruction to help plan surgery. The technique may also be of value in avoiding laparotomy in poor risk jaundice patients since failure to enter a duct suggests hepatocellular disease. Careful attention to technique is essential for the success and safety of the procedure. The procedure is usually performed under local anesthesia following analgesic premedication.
Depending upon the caliber of the biliary tree, a dose of 20 to 40 mL is generally sufficient to opacify the entire ductal system. If desired, the contrast agent may be diluted 1:1 with Sodium Chloride injection USP using strict aseptic procedures.
As the needle is advanced or withdrawn, a bile duct may be located by frequent aspiration for bile or mucus. Before the dose is administered, as much bile as possible is aspirated. The injection may be repeated for exposures in different planes and repositioning of the patient, if necessary, should be done with care. If a duct is not readily located by aspiration, successive small doses of 1 to 2 mL of the medium are injected into the liver as the needle is gradually withdrawn, until a duct is visualized by x-ray.
If no duct can be located after 3 or 4 attempts, the procedure should be terminated. Inability to enter a duct by a person experienced in the technique is generally considered to be strongly suggestive of hepatocellular disease.
ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY
Endoscopic retrograde cholangiopancreatography (ERCP) is indicated in carefully selected patients with known or suspected pancreatic or biliary tract disease when other diagnostic procedures have failed to provide the necessary diagnostic information. Prior to the development of ERCP, x-ray examination of the pancreatic ducts could only be obtained at laparotomy.
Precautions
Endoscopic retrograde cholangiopancreatography should only be performed by personnel skilled and experienced with the procedure, and careful attention to technique is essential for the success and safety of the procedure. Fluoroscopy is mandatory during injection to prevent over distention of the duct systems.
Adverse Reactions
Adverse reactions that have occurred which are attributable to either the procedure or to Conray, include nausea, vomiting, fever, severe abdominal pain, duodenal wall intravasation, septicemia, pancreatitis and perforation of the common bile duct associated with pathology.
Dosage and Administration
The procedure is usually performed following pharyngeal anesthesia and analgesic or sedative premedication. Duodenal motility may be controlled in patients with active duodenal peristalsis with an appropriate antiperistaltic agent.
The contrast medium should be injected slowly under fluoroscopic control employing the minimal dose that is adequate to visualize the common bile duct, the pancreatic duct, or both duct systems. The dosage will vary greatly depending on the pathological findings and can range from 10 to 100 mL for visualization of the common bile duct; and from 2 to 10 mL for visualization of the pancreatic duct.
Following the procedure, the patient should be kept under close observation for 24 hours.
CONTRAST ENHANCEMENT OF COMPUTED TOMOGRAPHIC (CT) BRAIN IMAGING
Tumors
Conray may be useful to enhance the demonstration of the presence and extent of certain malignancies such as: gliomas including malignant gliomas, glioblastomas, astrocytomas, oligodendrogliomas and gangliomas; ependymomas; medulloblastomas; meningiomas; neuromas; pinealomas; pituitary adenomas; craniopharyngiomas; germinomas; and metastatic lesions.
The usefulness of contrast enhancement for the investigation of the retrobulbar space and in cases of low grade or infiltrative glioma has not been demonstrated.
In cases where lesions have calcified, there is less likelihood of enhancement. Following therapy, tumors may show decreased or no enhancement.
Non-Neoplastic Conditions
The use of Conray may be beneficial in the image enhancement of non-neoplastic lesions. General infarctions of recent onset may be better visualized with the contrast enhancement, while some infarctions are obscured if contrast media are used. The use of iodinated contrast media results in contrast enhancement in about 60% of cerebral infarctions studied from one to four weeks from the onset of symptoms.
Sites of active infection may also be enhanced following contrast medium administration.
Arteriovenous malformations and aneurysms will show contrast enhancement. In the case of these vascular lesions, the enhancement is probably dependent on the iodine content of the circulating blood pool.
The opacification of the inferior vermis following contrast medium administration has resulted in false positive diagnoses in a number of normal studies.
Patient Preparation
No special patient preparation is required for contrast enhancement of CT brain scanning. However, it is advisable to ensure that patients are well hydrated prior to examination.
Usual Dosage
The usual dosage in adults and children is 2 mL/kg (1 mL/lb) by intravenous administration, not to exceed a total dose of 150 mL. In most cases, scanning may be performed immediately after completion of administration; however, when fast scanning equipment (less than 1 minute) is used, consideration should be given to waiting approximately 5 minutes to allow for maximum contrast enhancement.
CRANIAL COMPUTERIZED ANGIOTOMOGRAPHY
Conray may be administered for cranial computerized angiotomography when necessary to visualize the cerebral vessels to detect cerebrovascular lesions and to evaluate the anatomical relationship between the cerebral blood vessels and other parenchymal or space occupying lesions.
Usual Dosage
Conray may be administered by intravenous bolus injection, or by bolus injection followed by rapid infusion.
For bolus injection, the usual dose in adults and children is 0.5 to 1.0 mL/kg at an injection rate of 2 mL/second with scanning begun immediately after administration. This dose may be repeated as necessary. The total dose per procedure should not exceed 200 mL, and in children the total dose is reduced in approximate proportion to age and body weight.
In adults, when the combination bolus and infusion technique is used, a 50 mL bolus injection followed by a rapid infusion of 150 mL may be given or a 100 mL bolus injection followed by a rapid infusion of 100 mL may be used. Scanning is begun immediately after the bolus administration. In children, the dose is reduced in approximate proportion to age and body weight.
CONTRAST ENHANCEMENT IN BODY COMPUTED TOMOGRAPHY1
Conray may be administered when necessary to visualize vessels and organs in patients undergoing CT of the chest, abdomen and pelvis.
Patient Preparation
No special patient preparation is required for contrast enhancement in body CT. In patients undergoing abdominal or pelvic examination, opacification of the bowel may be valuable in scan interpretation.
Precautions
In addition to the general precautions previously described, it is advisable to ensure that patients are adequately hydrated prior to examination. Patient motion, including respiration, can markedly affect image quality, therefore, patient cooperation is essential. The use of an intravascular contrast medium can obscure tumors in patients undergoing CT evaluation of the liver resulting in a false negative diagnosis. Dynamic CT scanning is the procedure of choice for malignant tumor enhancement (see CLINICAL PHARMACOLOGY).
Usual Dosage
Conray may be administered by bolus injection, by rapid infusion or by a combination of both.
For vascular opacification, a bolus injection of 25 to 50 mL may be used, repeated as necessary. When prolonged arterial or venous phase enhancement is required and for the enhancement of specific lesions, a rapid infusion of 150 mL may be used. In some instances, a 100 to 150 mL infusion may be employed to define the area of interest followed by bolus injections of 20 to 50 mL to clarify selected scans.
INTRAVENOUS DIGITAL SUBTRACTION ANGIOGRAPHY
Intravenous digital subtraction angiography (IV DSA) is a radiographic modality which allows dynamic imaging of the arterial system following intravenous injection of iodinated x-ray contrast media through the use of image intensification, enhancement of the iodine signal and digital processing of the image data. Temporal subtraction of the images obtained during the “first arterial pass” of the injected contrast medium injection yield images which are devoid of bone and soft tissue.
Areas that have been most frequently examined by intravenous DSA are the heart, including coronary by-pass grafts; the pulmonary arteries; the arteries of the brachiocephalic circulation; the aortic arch; the abdominal aorta and its major branches including the celiac, mesenterics and renal arteries; the iliac arteries; and the arteries of the extremities.
Patient Preparation
No special patient preparation is required for intravenous digital subtraction angiography. However, it is advisable to ensure that patients are well hydrated prior to examination.
Precautions
In addition to the general precautions previously described, the risks associated with IV DSA are those usually attendant with catheter procedures and include intramural injections, vessel dissection and tissue extravasation. Small test injections of contrast medium made under fluoroscopic observation to ensure the catheter tip is properly positioned, and in the case of peripheral placement that the vein is of adequate size, will reduce this potential.
Patient motion, including respiration and swallowing, can result in marked image degradation yielding non-diagnostic studies. Therefore, patient cooperation is essential.
Usual Dosage
Conray may be injected either centrally, into the superior or inferior vena cava, or peripherally into an appropriate arm vein. For central injections, catheters may be introduced at the antecubital fossa into either the basilic or cephalic vein or at the leg into the femoral vein and advanced to the distal segment of the corresponding vena cava. For peripheral injections, the catheter is introduced at the antecubital fossa into the appropriate size arm vein. In order to reduce the potential for extravasation during peripheral injection, a catheter of approximately 20 cm in length should be employed.
Depending on the area to be imaged, the usual dose range is 20 to 40 mL. Injections may be repeated as necessary.
Central catheter injections are usually made with a power injector with an injection rate of between 10 and 30 mL/second. When making peripheral injections, rates of 12 to 20 mL/second should be used, depending on the size of the vein. Also, since contrast medium may remain in the arm vein for an extended period following injection, it may be advisable to flush the vein, immediately following injection with an appropriate volume (20 to 25 mL) of 5% Dextrose in water or normal saline.
ARTERIAL DIGITAL SUBTRACTION ANGIOGRAPHY
Arterial digital subtraction angiography provides images similar in quality to conventional film-screen systems. The advantages of arterial DSA when compared to standard film angiography include: the use of less contrast medium; the use of lower concentrations for some procedures; a decreased need for selective arterial catheterization reducing the possibility of dislodging atheromatous plaques or significantly reducing the blood flow in the artery; and a shortened examination time. The limitations of arterial DSA include: reduced spatial resolution; limited field size; and the inability to conduct simultaneous biplane examinations.
Patient Preparation
No special patient preparation is required for arterial DSA. However, it is advisable to ensure that patients are well hydrated prior to examination.
Precautions
In addition to the general precautions described, the risks associated with arterial DSA are those usually attendant with catheter procedures. Following the procedure, gentle pressure hemostasis is required, followed by observation and immobilization of the limb for several hours to prevent hemorrhage from the site of arterial puncture.
Usual Dosage
The following dosage schedule for adults should serve only as a guide since the volume administered, the concentration selected and the flow rate will be determined by the resolution of the equipment being used. As a general rule, the volume used and the flow rates for arterial DSA are 50% or less than that used for conventional film arteriography. Diagnostic studies have been obtained using Conray undiluted (28.2% iodine), diluted 1:1 (14.1% iodine), and diluted 1:2 (9.4% iodine). Sodium Chloride Injection USP or Water for Injection USP may be used for dilution.
The following doses, equivalent in iodine content to undiluted Conray, have been used.
| Carotid or vertebral arteries: | 3-8 mL |
| Aortic Arch: | 15-25 mL |
| Subclavian and brachial arteries: | 5-15 mL |
| Major branches of the aorta: | 5-20 mL |
| Lumbar aorta (bifurcation): | 10-25 mL |
How is Conray supplied
| Conray® Glass Vials/Bottles | NDC Number |
| 25x30 mL vials | 0019-0953-23 |
| 25x50 mL vials | 0019-0953-05 |
| 12x100 mL bottles | 0019-0953-10 |
| 12x150 mL bottles | 0019-0953-50 |
Storage
Store below 30°C (86°F). Exposing this product to very cold temperatures may result in crystallization of the salt. If this occurs, the container should be brought to room temperature. Shake vigorously to assure complete dissolution of any crystals. The speed of dissolution may be increased by heating with circulating warm air. Before use, examine the product to assure that all solids are redissolved, and that the container and closure have not been damaged.
This preparation is sensitive to light and must be protected from strong daylight or direct exposure to the sun.
As with all contrast media, glass containers should be inspected prior to use to ensure that breakage or other damage has not occurred during shipping and handling. All containers should be inspected for closure integrity. Damaged containers should not be used.
Reference
1 Young, S. W., Turner, R.J., Castellino, R. A.: “A strategy for the contrast enhancement of malignant tumors using dynamic computed tomography and intravascular pharmacokinetics,” Radiology, 137:137-147, October 1980.
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
GBT 0953A223
Revised 02/2023
Package Label Principal Display Panel - 150mL Bottle
For Intravascular Use
Sterile Solution
Conray®
150mL
NDC 0019-0953-50
Iothalamate Meglumine Injection USP 60%
282 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx Only
Protect from light • Store below 30°C (86°F).
Each mL contains 600 mg iothalamate meglumine, 0.09 mg edetate calcium disodium as a stabilizer, and 0.125 mg monobasic sodium phosphate as a buffer.
Single dose container • Discard unused portion
Usual Dosage: See Package Insert.
10390419
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616Made in USA
| CONRAY
iothalamate meglumine injection |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Liebel-Flarsheim Company LLC (057880002) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LIEBEL-FLARSHEIM COMPANY LLC | 109024984 | ANALYSIS(0019-0953) , MANUFACTURE(0019-0953) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Justesa Imagen, S.A.U. | 477020325 | API MANUFACTURE(0019-0953) | |
More about Conray (iothalamate)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Latest FDA alerts (2)
- Side effects
- Dosage information
- During pregnancy
- Drug class: ionic iodinated contrast media
- Breastfeeding
