Adenovirus Type 4-and Type-7 Vaccine Live: Package Insert / Prescribing Info
Package insert / product label
Dosage form: tablet, enteric coated
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Jul 21, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral
Enteric Coated Tablets for Oral Administration
Initial U.S. Approval: 2011
Indications and Usage for Adenovirus Type 4-and Type-7 Vaccine Live
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is a vaccine indicated for active immunization for the prevention of febrile acute respiratory disease caused by Adenovirus Type 4 and Type 7. Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is approved for use in military populations 17 through 50 years of age. (1)
Adenovirus Type 4-and Type-7 Vaccine Live Dosage and Administration
- A single vaccine dose is administered orally as two tablets: one tablet of Adenovirus Type 4 and one tablet of Adenovirus Type 7. (2)
- Each of the two tablets should be swallowed whole. The tablets should not be chewed or crushed to avoid releasing the live adenovirus in the upper respiratory tract.
- Postpone administration in vaccinees with vomiting and/or diarrhea.
Dosage Forms and Strengths
A single oral dose consists of one Adenovirus Type 4 tablet and one Adenovirus Type 7 tablet. (3)
Contraindications
Warnings and Precautions
- Safety and effectiveness have not been evaluated in persons with primary or acquired immunodeficiency states. (5.1)
- Vaccinees and individuals who come in close contact with vaccinees may be exposed to the vaccine viruses shed in the stool for up to 28 days. Proper personal hygiene can minimize this risk. (5.2)
- Vaccinees should exercise caution when in close contact with children less than 7 years of age, immunocompromised individuals and pregnant women during the 28 days following vaccination. (5.2)
- Use effective contraception for 6 weeks after vaccination to avoid pregnancy. (5.3)
Adverse Reactions/Side Effects
The most common (≥ 5%) systemic adverse reactions observed in clinical trials were upper respiratory tract infections, headache, nasal congestion, pharyngolaryngeal pain, cough, arthralgia, nausea, abdominal pain, diarrhea, and vomiting. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
Use In Specific Populations
Administration during pregnancy is contraindicated (4.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2022
Full Prescribing Information
1. Indications and Usage for Adenovirus Type 4-and Type-7 Vaccine Live
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is a vaccine indicated for active immunization for the prevention of febrile acute respiratory disease caused by Adenovirus Type 4 and Type 7. Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is approved for use in military populations 17 through 50 years of age.
2. Adenovirus Type 4-and Type-7 Vaccine Live Dosage and Administration
A single vaccine dose is administered orally as two tablets: one tablet of Adenovirus Type 4 and one tablet of Adenovirus Type 7. (2)
The tablets should be swallowed whole, without chewing, to avoid releasing the virus in the upper respiratory tract [See Mechanism of Action (12.1)].
Postpone administration to individuals with vomiting and/or diarrhea because the effectiveness of the vaccine depends upon the multiplication of orally administered live adenovirus within the intestinal tract [See Mechanism of Action (12.1)].
3. Dosage Forms and Strengths
A single vaccine dose consists of two tablets: one tablet of Adenovirus Type 4 and one tablet of Adenovirus Type 7.
4. Contraindications
4.1 Pregnancy
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral should not be administered to pregnant females [See Pregnancy (8.1)]. It is not known whether Adenovirus Type 4 and Type 7 Vaccine, Live, Oral can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Naturally occurring infection with adenoviruses has been associated with fetal harm. Pregnancy should be avoided for 6 weeks following receipt of vaccine.
4.2 Severe Allergic Reaction
Severe allergic reaction (e.g., anaphylaxis) to any component of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is a Contraindication [See Description (11)].
4.3 Inability to Swallow
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral should not be administered to individuals incapable of swallowing each entire tablet, whole, without chewing. Chewing a tablet could expose the upper respiratory tract to live adenovirus leading to disease [See Mechanism of Action (12.1)].
5. Warnings and Precautions
5.1 Altered Immunocompetence
The safety and effectiveness of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral in immunocompromised individuals has not been evaluated.
5.2 Shedding and Transmission
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral contains live viruses that are shed in the stool and can cause disease if transmitted.
People who come in close contact with those who were vaccinated, including other vaccinees, may be exposed to the virus present in the stool and may develop disease [See Pharmacodynamics (12.2)].
Persons vaccinated with Adenovirus Type 4 and Type 7 Vaccine, Live, Oral should exercise caution when in close contact with children less than 7 years of age and immunocompromised individuals such as those with HIV infection and cancer, or those receiving immunosuppressive therapy during the 28 day period of viral shedding following the vaccination [See Use in Specific Populations (8.3) and Pharmacodynamics (12.2)].
Vaccinees should exercise caution when in close contact with pregnant women during the 28 day period of shedding because fetal harm may result if pregnant women are exposed to adenovirus [See Pregnancy (8.1) and Pharmacodynamics (12.2)].
5.3 Planning for Pregnancy
Use effective contraception for 6 weeks after vaccination to avoid pregnancy [See Pregnancy (8.1)].
5.4 Human Serum Albumin
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral contains albumin, a derivative of human blood. It is present at concentrations of <0.3 mg/tablet. Based on effective donor screening, and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmissions of viral diseases or CJD have ever been identified for human albumin.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to the rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Multicenter Safety and Efficacy Trial
Safety of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral was evaluated in a multicenter, double-blind, randomized, placebo-controlled study that enrolled 3031 subjects who received vaccine and 1009 subjects who received placebo (lactose tablets). The study was conducted in healthy male (63%) and female (37%) active duty US Army and Navy military recruits during their basic training. The population had a mean age of 21 years, with an age range of 17 to 42 years. Race was 62% Caucasian, 18% African-American, 11% Hispanic, 3% Asian and 6% other. Subjects in both groups were administered other vaccines concomitantly with Adenovirus Type 4 and Type 7 Vaccine, Live, Oral. The specific vaccines that each subject received varied and were dependent on their immunization history. The vaccines that were co-administered included Hepatitis A Vaccine, Inactivated (Merck & Co., Inc.), Hepatitis A Inactivated and Hepatitis B (Recombinant) Vaccine (GlaxoSmithKline Biologicals), Hepatitis B Vaccine (Recombinant) (Merck & Co., Inc.), Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant (Merck & Co., Inc.), Influenza Vaccine, Live, Intranasal (MedImmune, LLC), Influenza Virus Vaccine (Sanofi Pasteur, Inc.), Measles, Mumps, and Rubella Virus Vaccine Live (Merck & Co., Inc.), Meningococcal (Groups A, C, Y and W-135) Polysaccharide, Diphtheria Toxoid Conjugate Vaccine (Sanofi Pasteur, Inc.), Meningococcal Polysaccharide Vaccine (Groups A, C, Y and W-135 Combined) (Sanofi Pasteur, Inc.), Poliovirus Vaccine Inactivated (Sanofi Pasteur, SA), Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed (Sanofi Pasteur, Ltd.), Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed (GlaxoSmithKline Biologicals), Typhoid Vi Polysaccharide Vaccine (Sanofi Pasteur, SA), Varicella Virus Vaccine Live (Merck & Co., Inc.), Yellow Fever Vaccine (Sanofi Pasteur, Inc.).
Serious Adverse Events
No deaths were reported during the multicenter safety and efficacy trial.
Serious adverse events in vaccine recipients included hematuria, gastroenteritis, febrile gastroenteritis, gastritis, pneumonia, and hematochezia.
Fifty-seven serious adverse events (SAEs) were reported during the six month study period with 39 reported between 0 and 56 days following treatment and 18 reported during the 56 to 180 day follow-up period. Thirty-five subjects (1.2%) who received vaccine (25 between 0 and 56 days from the date of vaccination, 10 during the 56 to 180 day follow-up period) and 12 subjects (1.2%) who received placebo (9 between 0 and 56 days from the date of treatment, 3 during the 56 to 180 day follow-up period) experienced at least one SAE. The SAEs occurring between Day 0 and Day 56 post-vaccination in the vaccine group, possibly associated with the receipt of the vaccine product as determined by the investigator, were as follows: one subject with hematuria and gastroenteritis (at 9 days post vaccination), one subject with febrile gastroenteritis (at 4 days post vaccination, one subject with gastritis (at 23 days post vaccination), and one subject with pneumonia (at 23 days post vaccination); one SAE (hematochezia) in the vaccine group occurred during the 56 to 180 day follow-up period and was determined to be possibly related to the vaccine product. A placebo recipient developed febrile acute respiratory disease where adenovirus Type 4 vaccine strain was detected from posterior pharyngeal and tonsillar swabbing and characterized by serotyping and polymerase chain reaction analysis [See Warnings and Precautions: Shedding and Transmission (5.2)].
Overall, the percentage of subjects who experienced at least one adverse event during the 56 day study period was 91.2% in the Adenovirus Type 4 and Type 7 Vaccine, Live, Oral group compared to 93.9% in the placebo group. No subject in either treatment arm discontinued the study due to an adverse event. Adverse reactions were captured on a 2-Week Daily Diary (for a minimum of the first 780 subjects) or a 1-Week Daily Diary (for all remaining subjects) and were also reported at each study visit up to Day 56 after vaccination. Any reported AEs for Days 0-14 for the safety cohort and for Days 0-7 for the remaining subjects were defined as "solicited" because they were almost exclusively recorded directly by the subject from a pre-defined diary checklist. Although pyrexia was defined as “solicited,” it was not on the pre-defined diary checklist. Any AEs reported spontaneously as part of the regular study visit or during a spontaneous visit to the clinic, for Days 15-56 for the safety cohort and Days 8-56 for the remaining subjects were designated as "non-solicited."
Solicited Adverse Reactions
The following solicited adverse reactions were collected through daily diaries: stuffy nose, cough, sore throat, stomach pain, headache, diarrhea, nausea, and joint pain (within 14 days post enrollment for subjects in the initial safety cohort (n=878) and within 7 days post enrollment all subjects (n= 4040) for the rest of safety population). Those solicited adverse reactions reported by ≥ 5 % of subjects in either the vaccine or placebo treatment groups are presented in Table 1.
| Adverse Reaction* | Adenovirus Type 4 and Type 7 Vaccine, Live, Oral | Placebo |
||||||
| 0-7 Days N = 3031 | 8-14 Days N = 660 | 0-7 Days N = 1009 | 8-14 Days N = 218 |
|||||
| n | % | n | % | n | % | n | % |
|
| Headache | 894 | 29.5 | 38 | 6.5 | 310 | 30.7 | 11 | 5.6 |
| Nasal Congestion (Stuffy Nose) | 463 | 15.3 | 49 | 8.4 | 141 | 14.0 | 12 | 6.2 |
| Pharyngolaryngeal Pain (Sore Throat) | 391 | 12.9 | 72 | 12.3 | 124 | 12.3 | 24 | 12.3 |
| Cough | 375 | 12.4 | 59 | 10.1 | 130 | 12.9 | 14 | 7.2 |
| Nausea | 412 | 13.6 | 29 | 5.0 | 137 | 13.6 | 11 | 5.6 |
| Diarrhea | 310 | 10.2 | 18 | 3.1 | 84 | 8.3 | 10 | 5.1 |
*MedDRA Preferred Term
Pyrexia (temp ≥ 100.5°F) within 7 days, was reported to occur in 1.4% (42/3030) of vaccine recipients and 0.5% (5/961) of placebo recipients who were not diagnosed with ARD. During the 8-14 days post vaccination, rates of pyrexia were 0.6% (4/659) and 1.1% (2/170) in vaccine and placebo recipients, respectively.
Non-Solicited Adverse Reactions
Non-solicited adverse reactions that occurred Days 15-56 in the safety cohort and Days 8-56 for all remaining subjects, reported by ≥ 5 % of subjects in either the vaccine or placebo treatment groups, are presented in Table 2.
| Adverse Reaction* |
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral
|
Placebo
|
||
| N | % | N | % |
|
| Upper Respiratory Tract Infection | 1135 | 37.5 | 397 | 39.4 |
| Arthralgia | 524 | 17.3 | 180 | 17.8 |
| Abdominal Pain Upper | 443 | 14.6 | 157 | 15.6 |
| Headache | 330 | 10.9 | 148 | 14.7 |
| Cough | 257 | 8.5 | 91 | 9.0 |
| Pharyngolaryngeal Pain | 253 | 8.4 | 73 | 7.2 |
| Nasal Congestion | 229 | 7.6 | 73 | 7.2 |
| Vomiting | 160 | 5.3 | 55 | 5.5 |
| Chills | 77 | 2.5 | 51 | 5.1 |
*MedDRA Preferred Term
Less common (less than 5%) adverse reactions reported in the clinical trial in military recruits receiving Adenovirus Type 4 and Type 7 Vaccine, Live, Oral, versus placebo, respectively included rhinorrhea (128 [4.22%] vs. 25 [2.48%]), pain in extremity (130 [4.29%] vs. 37 [3.67%]), and pyrexia (fever greater than or equal to 100.5 °F) (126 [4.16%] vs. 49 [4.86%]).
Safety and Immunogenicity Trial
Five SAEs were reported among the 58 subjects in the safety and immunogenicity trial. Two SAEs occurred among the vaccine recipients: one case of pneumonia reported on Day 33 of the follow-up period, and a report of appendicitis occurring on Day 118 of follow-up period. Three SAEs were reported among placebo recipients: one case of pneumonia on Day 10 and one case of upper respiratory infection reported on Day 14, and a right thigh abscess reported at Day 91.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Immune System Disorders: Hypersensitivity reactions (including anaphylaxis)
Nervous System Disorders: Guillain-Barré syndrome
Related/similar drugs
7. Drug Interactions
7.1 Concomitant Vaccine Administration
In clinical studies Adenovirus Type 4 and Type 7 Vaccine, Live, Oral was administered concurrently with other vaccines [See Adverse Reactions (6.1)]. Data were not available to assess whether Adenovirus Type 4 and Type 7 Vaccine, Live, Oral interferes with the immune response to the other vaccines.
7.2 Immunosuppressive Therapies
There are no data regarding the use of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral concomitantly with immunosuppressive therapies, e.g., irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses) [See Warnings and Precautions: Altered Immunocompetence (5.1)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Do not administer Adenovirus Type 4 and Type 7 Vaccine, Live, Oral to pregnant females [See Contraindications (4.1)]. It is not known whether Adenovirus Type 4 and Type 7 Vaccine, Live, Oral can cause fetal harm when administered to a pregnant woman. Naturally occurring infection with adenoviruses has been associated with fetal harm.
Pregnancy should be avoided for 6 weeks following receipt of vaccine.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor and Delivery
There is no information regarding the effect of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral on labor and delivery. Fecal shedding during delivery may result in vaccine virus transmission to the newborn infant.
Data
Human Data
Five pregnancies were reported among women enrolled in the multicenter safety and efficacy trial of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral. Four of the subjects (3 vaccine recipients and 1 placebo recipient) were estimated to have conceived 2 to 13 days prior to vaccination. One subject (vaccine recipient) conceived approximately 21 weeks after vaccination. The deliveries to all five of the subjects were of healthy infants at estimated gestational ages between 36 and 40 weeks.
8.2 Lactation
Risk Summary
There are no data on the presence of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral in human milk, effects on milk production, or on the breastfed infant.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Adenovirus Type 4 and Type 7 Vaccine, Live, Oral and any potential adverse effects on the breastfed infant from Adenovirus Type 4 and Type 7 Vaccine, Live, Oral or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is contraindicated for use in pregnant women [See Pregnancy (4.1)]. Females of reproductive potential should have a pregnancy test prior to treatment with Adenovirus Type 4 and Type 7 Vaccine, Live, Oral.
Contraception
Advise females of reproductive potential to use effective contraception during treatment with Adenovirus Type 4 and Type 7 Vaccine, Live, Oral and for 6 weeks following receipt of vaccine.
11. Adenovirus Type 4-and Type-7 Vaccine Live Description
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral contains viable, selected strains of human adenovirus Type 4 and human adenovirus Type 7 prepared in human-diploid fibroblast cell cultures (strain WI-38). The virus strains have not been attenuated. The cells are grown and the virus growth maintained in Dulbecco’s Modified Eagle’s Medium, fetal bovine serum, and sodium bicarbonate. The virus is harvested, freed of particulate cellular material by filtration, formulated and dried by lyophilization. The dried virus material includes monosodium glutamate, sucrose, D-mannose, D-fructose, dextrose, human serum albumin, potassium phosphate, and plasdone C.
The final vaccine is composed of two tablets (one tablet of Adenovirus Type 4 and one tablet of Adenovirus Type 7) designed to pass intact through the stomach and release the live virus in the intestine. Each enteric-coated tablet contains an inner core tablet containing anhydrous lactose, microcrystalline cellulose, polacrilin potassium, magnesium stearate, and live adenovirus, either Type 4 or Type 7, at a potency of no fewer than 32,000 tissue-culture infective doses (4.5 log10 TCID50) per tablet. The outer tablet layer contains microcrystalline cellulose, magnesium stearate, and anhydrous lactose, with an enteric coating consisting of cellulose acetate phthalate, alcohol, acetone, and castor oil. The Type 7 tablet also contains FD&C Yellow #6 aluminum lake dye.
12. Adenovirus Type 4-and Type-7 Vaccine Live - Clinical Pharmacology
12.1 Mechanism of Action
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is a live oral vaccine that replicates in the intestinal tract and induces immunity in persons with low or no pre-existing neutralizing antibodies.
12.2 Pharmacodynamics
Vaccine Virus Shedding
Fecal shedding of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral strain viruses was evaluated in a safety and immunogenicity study of 58 subjects (30 vaccine recipients and 28 placebo recipients) [See Clinical Studies (14)]. Stool or rectal swabs and throat swabs were collected on Day 0, 7, 14, 21, 28 and 56. Vaccine virus strains were shed in the stool as early as day 7 following vaccination. Eight of 30 vaccine recipients (27%) tested positive at least once for Adenovirus Type 4 fecal shedding; 18 of 30 vaccine recipients (60%) tested positive for Adenovirus Type 7 fecal shedding. No adenovirus shedding was detectable in any subject by 28 days following vaccination. Vaccine strain virus was not detected in the throat of any subject.
14. Clinical Studies
14.1 Multicenter Safety and Efficacy Trial
A multicenter, double-blind, randomized, placebo-controlled study in US military recruits evaluated the safety and efficacy of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral to prevent wild Type 4 adenovirus-associated febrile acute respiratory disease (ARD) and to induce neutralizing antibody to Type 7 adenovirus. A seroconversion endpoint rather than prevention of clinical disease was used to assess efficacy of the Type 7 adenovirus vaccine component of this product as the incidence of febrile ARD due to Type 7 adenovirus was not anticipated to be high enough to permit a meaningful statistical assessment of the clinical effect of this vaccine component. Subjects were randomized to either the vaccine group or the placebo group in a 3:1 ratio. 4041 subjects were randomized, and 4040 subjects were analyzed. Females and males aged 17 or older and in good physical health were included in the study. No subjects were immunosuppressed or being treated with systemic immunosuppressive therapy. Baseline serology data is presented in Table 3.
| Vaccine N=3031 | Placebo N=1009 | Total
N=4040 |
|
| Type 4 Titer | n (%) | n (%) | n (%) |
| Negative* | 1906 (63%) | 678 (67%) | 2584 (64%) |
| Positive** | 1123 (37%) | 331 (33%) | 1454 (36%) |
| Type 7 Titer | |||
| Negative* | 1159 (38%) | 377 (37%) | 1536 (38%) |
| Positive** | 1870 (62%) | 632 (63%) | 2502 (62%) |
| * Titer value was <1:4 at visit 0. ** Titer value was ≥1:4 at visit 0. |
|||
Adenovirus Type 4 Efficacy and Immunogenicity
Febrile Acute Respiratory Disease (ARD): The results for the primary analysis of vaccine efficacy (VE) for adenovirus Type 4 and rate of wild Type 4 febrile ARD, are summarized in Table 4. Cases were defined as subjects with one or more clinical signs and symptoms of ARD (mild to severe: sore throat, cough, rhinorrhea, nasal congestion, rales or rhonchi), an oral temperature > 100.5°F, and throat culture positive for wild Type 4 adenovirus infection. Vaccine type Adenovirus was distinguished from wild type by a Polymerase Chain Reaction (PCR) assay.
| Adeno Type 4 ARD case | Statistic | Vaccine N=3031 | Placebo
N=1009 |
| Yes | n | 1* | 48 |
| VE (95% CI)§ | 99.3% (96.0%, 99.9%) |
||
| *one additional subject met the case definition but had non-vaccine serotype (B3) adenovirus |
|||
| §Vaccine efficacy (VE) defined as: VE = 1 - RR, where RR = P(vaccine)/P(placebo) was the relative risk of ARD attack in subjects who received vaccine compared to placebo; 2-sided confidence interval, by using exact statistical methods. |
|||
Seroconversion Rate: Adenovirus Type 4 seroconversion rate is presented in Table 5. Seroconversion is defined as the development of a Type 4 neutralizing antibody titer of greater or equal to 1:8 at Day 26 after vaccination in subjects whose baseline titer was less than 1:4.
|
Adenovirus Type 4 and Type 7 Vaccine, Live Oral | Placebo |
||||
| N |
Seroconversion | N | Seroconversion |
||
| n | % (95% CI) | n | % (95% CI) |
||
| 1841 | 1739 | 94.5% ( 93.4%, 95.5% ) | 653 | 69 | 10.6 % ( 8.2%, 12.9% ) |
Adenovirus Type 7 Immunogenicity
Febrile Acute Respiratory Disease: No Type 7 adenovirus-associated febrile or afebrile ARD cases were reported for either placebo or vaccine groups. This was expected given the estimated attack rate of adenovirus Type 7 in the military base training setting at the time of the study. Seroconversion was the primary evaluation of Type 7 effectiveness.
Seroconversion Rate: Results for the primary analysis of adenovirus Type 7 efficacy, seroconversion rate at Day 26, are summarized in Table 6. Seroconversion is defined as the development of a Type 7 neutralizing antibody titer of greater or equal to 1:8 at Day 26 after vaccination in subjects whose baseline titer was less than 1:4.
|
Adenovirus Type 4 and Type 7 Vaccine, Live Oral | Placebo |
||||
| N |
Seroconversion
| N | Seroconversion |
||
| n | % (95% CI) | n | % (95% CI) |
||
| 1120 | 1051 | 93.8% (92.4%, 95.2% ) | 359 | 19 | 5.3% (3.0%, 7.6% ) |
15. References
- Van den Veyver et al. 1998. Detection of intrauterine viral infection using the polymerase chain reaction. Mol Genet Metab, 63: 85-95
- Calvin et al. 2000. Fatal intrauterine adenoviral endomyocarditis with aortic and pulmonary valve stenosis: diagnosis by polymerase chain reaction. Hum Pathol. 31(11):1433-5
- Ranucci-Weiss et al. 1998. Intrauterine adenoviral infection associated with fetal non-immune hydrops. Prenat. Diagn. 18(2):182-5
- Baschat et al., 2003. Is adenovirus a fetal pathogen? Am J Obstet Gynecol. 189(3):758-63.
- Fields Virology, Fifth Edition, 2007, p2409
16. How is Adenovirus Type 4-and Type-7 Vaccine Live supplied
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Enteric Coated Tablets is packaged in a carton of two bottles of 100 tablets of each component of the vaccine:
- Adenovirus Type 4 Component of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral enteric coated tablet, a white to off-white, round, coated tablet with stylized b and a 4 imprinted on one side, for oral administration NDC 51285-174-02
- Adenovirus Type 7 Component of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral enteric coated tablet, a light peach, round, coated tablet with stylized b and a 7 imprinted on one side, for oral administration NDC 51285-175-02
- Supplied as a single carton containing 1 x 100 tablets Adenovirus Type 4 Component and 1 x 100 tablets Adenovirus Type 7 Component NDC 51285-138-50
Store refrigerated between 2° and 8° C (35° and 46° F). Do not freeze. Keep bottle tightly closed and protect from moisture. Do not remove desiccant canister from bottle.
17. Patient Counseling Information
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral is contraindicated for use in pregnant women.
Inform women to avoid becoming pregnant following vaccination for at least 6 weeks after vaccination to prevent the fetus from being exposed to adenovirus.
Instruct patients to swallow each tablet whole without chewing.
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral contains live virus that is shed in the stool for up to 28 days following vaccination and can cause disease if transmitted. It is given to individuals, undergoing intensive military training, who have limited contact with pregnant women, children under age seven and persons with compromised immune systems. To minimize the risk for transmitting and infecting others with the virus, instruct patients to take the following precautions during the 28-day period following vaccination:
- Observe proper personal hygiene, such as, frequent hand washing, especially following bowel movements.
- Caution is advised when in close contact with children less than 7 years of age, immunocompromised individuals (e.g., HIV infected, has cancer, or is receiving cancer treatments) and pregnant women because they may be more vulnerable to infection if exposed to the virus.
If anyone has any questions or concerns regarding this vaccine, they should speak with their healthcare provider.
Manufactured by:
Teva Women's Health, Inc.
West Chester, PA 19380
Manufactured for:
Teva Pharmaceuticals
Parsippany, NJ 07054
©2022 Teva Pharmaceuticals
Rev. 7/2022
Package/Label Display Panel, Type 4 Component

Adenovirus Type 4 Component of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Tablets 100s Label Text
NDC 51285-174-02
Rx only
Adenovirus Type 4 Component
Of Adenovirus Type 4 and
Type 7 Vaccine, Live, Oral
Enteric coated tablets,
For oral administration
NOT TO BE USED ALONE
Take with Adenovirus Type 7 Component
100 tablets
teva
Package/Label Display Panel, Type 7 Component

Adenovirus Type 7 Component of Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Tablets 100s Label Text
NDC 51285-175-02
Rx only
Adenovirus Type 7 Component
Of Adenovirus Type 4 and
Type 7 Vaccine, Live, Oral
Enteric coated tablets,
For oral administration
NOT TO BE USED ALONE
Take with Adenovirus Type 4 Component
100 tablets
teva
Package/Label Display Panel, Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Kit Carton, Part 1 of 2

Package/Label Display Panel, Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Kit Carton, Part 2 of 2
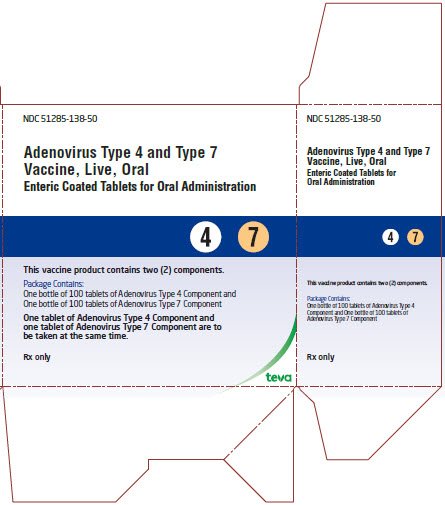
Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Tablets Kit Carton Text
NDC 51285-138-50
Adenovirus Type 4 and Type 7
Vaccine, Live, Oral
Enteric Coated Tablets for Oral Administration
- 4 7
This vaccine product contains two (2) components.
Package contains:
One bottle of 100 tablets of Adenovirus Type 4 Component and
One bottle of 100 tablets of Adenovirus Type 7 Component
One tablet of Adenovirus Type 4 Component and
one tablet of Adenovirus Type 7 Component are to
be taken at the same time.
Rx only
teva
| ADENOVIRUS TYPE 4 AND TYPE 7 VACCINE, LIVE
adenovirus type 4 and type 7 vaccine, live kit |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Teva Women's Health, Inc. (017038951) |
More about adenovirus vaccine
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: viral vaccines
