Umary USA Issues Voluntary Nationwide Recall of Unavy Ácido Hialurónico Caplets and Umovy Ácido Hialurónico Caplets Due to the Presence of Undeclared Drug Ingredients
Audience: Consumer
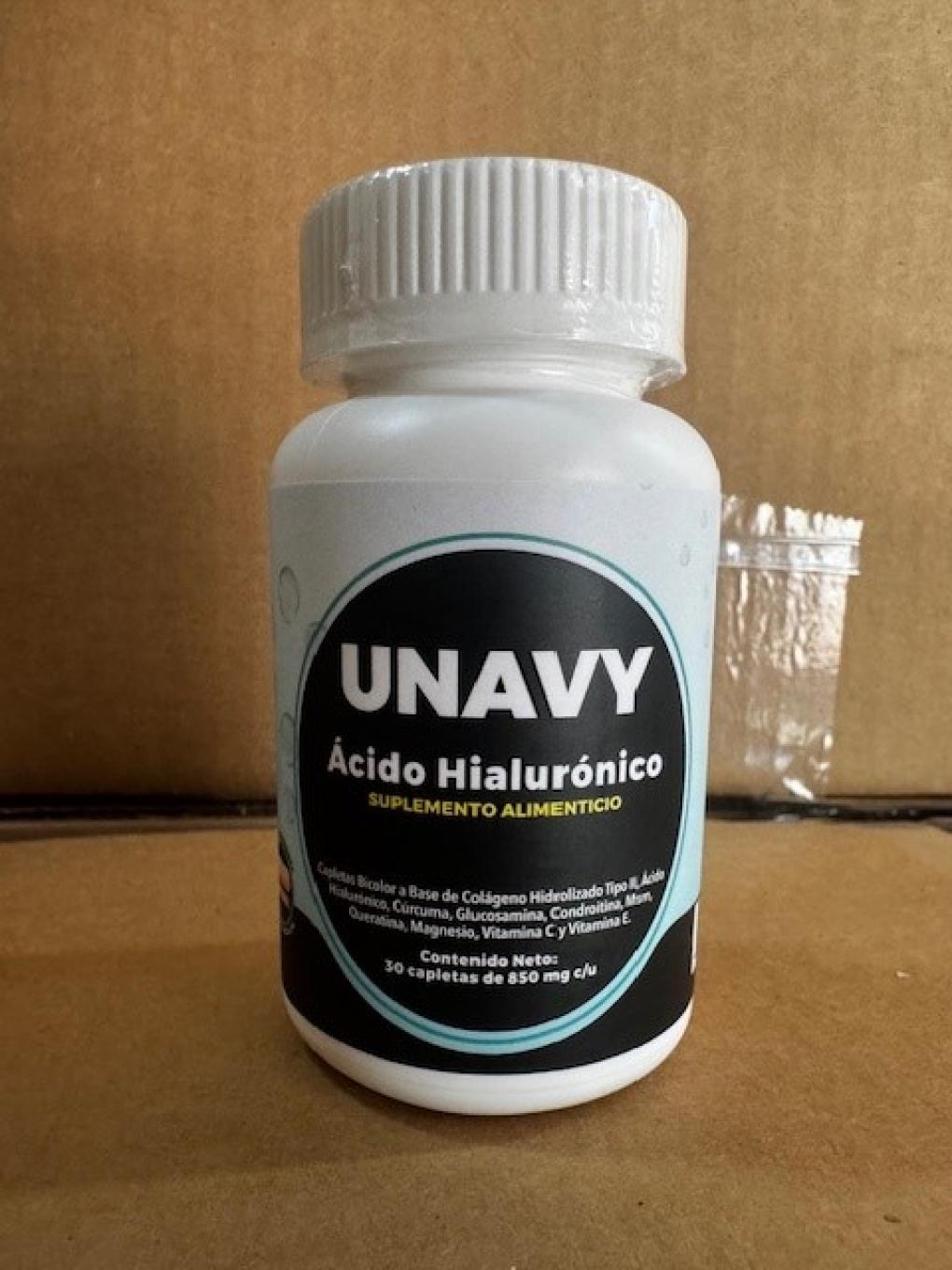
May 21 2025, Nogales, AZ, Umary USA is voluntarily recalling all lots of Unavy Ácido Hialurónico (30 caplets/850 mg) and Umovy Ácido Hialurónico (30 caplets/850 mg), to the consumer level. FDA laboratory analysis confirmed that these products are tainted with the drug ingredients Diclofenac, Dexamethasone and Omeprazole. Products containing Diclofenac, Dexamethasone or Omeprazole cannot be marketed as dietary supplements. Unavy Ácido Hialurónico and Umovy Ácido Hialurónico are unapproved new drugs for which safety and efficacy have not been established and, therefore, subject to recall.
Risk Statement: Dexamethasone is a corticosteroid commonly used to treat inflammatory conditions. Corticosteroid use can impair a person’s ability to fight infections and can cause high blood sugar levels, muscle injuries, psychiatric problems, and lead to cardiovascular events. When corticosteroids are taken for a prolonged period, or at high doses, they can suppress the adrenal gland (a disorder in which the adrenal glands do not produce enough hormones) and adverse consequences can range from limited adverse consequences to death. Additionally, abrupt discontinuation can cause withdrawal symptoms. Diclofenac is a non-steroidal anti-inflammatory drug (commonly referred to as NSAIDs). Consumption of undeclared diclofenac could result in serious adverse events that include cardiovascular, gastrointestinal, renal, and anaphylaxis in patients taking concomitant NSAIDs and/or anticoagulants, in those who have allergies to diclofenac, or those with underlying illnesses. Omeprazole is a proton pump inhibitor (commonly referred to as PPI) used to treat gastric (stomach) acid-related disorders. Consumption of undeclared omeprazole may mask stomach issues such as erosions, ulcers, and stomach cancer. Consumption of undeclared dexamethasone, diclofenac, and omeprazole can also interact with other medications and cause serious side effects. Umary- usa.com has not received any reports of adverse events related to this recall.
These products are marketed as dietary supplements for joint pain and arthritis. Unavy Ácido Hialurónico is packaged in a white plastic container with a black background label, and white and yellow writing on it. The bottle has 30 caplets/ 850 mg. The affected product includes all lots and expiration dates. Umovy Ácido Hialurónico is a black plastic bottle with a black label with white and blue lettering on the label. The bottle has 30 caplets/ 850mg. The affected product includes all lots and expiration dates. Product was distributed Nationwide via internet, exclusively via umary-usa.com.
Umary-usa.com is notifying its distributors and customers by Press release and email and is arranging for return and refund of all recalled products. Consumers who have any of these products, should immediately work with their physician and/or health care provider to safely discontinue use of these products. Consumers with questions regarding this recall can contact umary-usa.com at umaryusa2025@gmail.com, available seven days a week, 24 hours a day. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm1
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm2 or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.



.682fd3de138c2.jpg)
.682fd3efcdc81.jpg)
.682fd3feeda63.jpg)
Source: FDA
More news resources
- FDA Medwatch Drug Alerts
- Daily MedNews
- News for Health Professionals
- New Drug Approvals
- New Drug Applications
- Drug Shortages
- Clinical Trial Results
- Generic Drug Approvals
Subscribe to our newsletter
Whatever your topic of interest, subscribe to our newsletters to get the best of Drugs.com in your inbox.
