Sodium chloride FDA Alerts
The FDA Alerts below may be specifically about sodium chloride or relate to a group or class of drugs which include sodium chloride.
MedWatch Safety Alerts are distributed by the FDA and published by Drugs.com. Following is a list of possible medication recalls, market withdrawals, alerts and warnings.
Recent FDA Alerts for sodium chloride
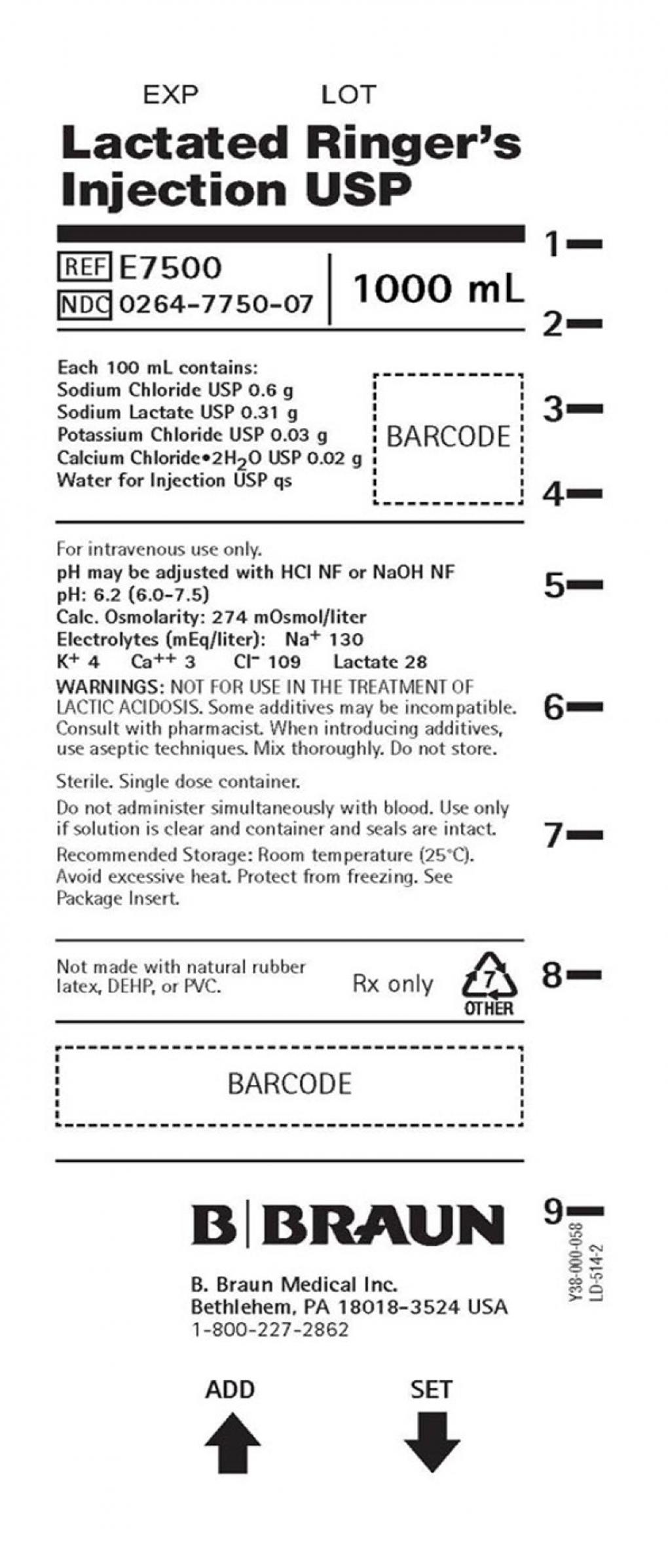
B. Braun Medical Issues Voluntary Nationwide Recall of Lactated Ringer’s Injection USP 1000 mL and 0.9% Sodium Chloride Injection USP 1000 mL Due to the Presence of Particulate Matter
BETHLEHEM, PA – August 19, 2025 – B. Braun Medical Inc. (B. Braun) is voluntarily recalling two lots of Lactated Ringers Injection USP 1000 mL, and 0.9% Sodium Chloride Injection USP 1000 mL to the hospital level due to the presence of particulate matter inside the container.
B. Braun has identified through complaints the potential for the product to contain particulate matter in solution. To date there have been no reports of serious injury, death or other adverse events associated with this issue. If the particulate matter is observed before use, a minor delay could occur while obtaining a replacement product. If the particulate matter is loose and the container is used on a patient, there is a potential for the particulate to be infused into the circulatory system. This could lead to patient harm that may require additional medical intervention and/or lead to permanent impairment or death.
The product has a reasonable probability of causing pulmonary emboli (blockage in pulmonary blood vessels), occlusions of other blood vessels (which can lead to tissue death and possible organ damage), and/or phlebitis (inflammation of the walls of veins, which may lead to clotting). Systemically, foreign particles infused intravenously can cause systemic activation of the immune system, organ dysfunction, and hemolysis (breakdown of blood cells). To date there have been no reports of serious injury, death or other adverse events associated with this issue.
0.9% Sodium Chloride Injection USP is indicated for extracellular fluid replacement, treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. Lactated Ringers Injection USP 1000 mL solution is indicated for use in adults and pediatric patients as a source of electrolytes and water for hydration. These products are packaged in boxes of 12 eaches. Additional details on the affected products are as follows:
|
Product |
NDC Number |
Product Description |
Lot |
Distribution |
Expiration |
Region |
|---|---|---|---|---|---|---|
|
E7500 |
0264-7750-07 |
Lactated Ringers Injection |
J4S807 |
26DEC2024 |
31MAY2027 |
US |
|
E8000 |
0264-7800-09 |
0.9% Sodium Chloride |
V3K770 |
15NOV2023 |
31JAN2026 |
US |
These products were distributed nationwide via distributors.
B. Braun is notifying its distributors and customers by certified mail and is arranging for return. of all recalled products. Distributors that have affected product which is being recalled should determine their current inventory of the affected items within inventory of their facility, cease use and distribution and quarantine product subject to recall. Affected product should not be destroyed.
Customers who have questions about this recall should contact our B. Braun’s Recalls Department at 844-903-6417 between 9 AM and 5 PM EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About B. Braun
B. Braun Medical Inc., a leader in infusion therapy and pain management, develops, manufactures, and markets medical products and services to the healthcare industry. Other key product areas include nutrition, pharmacy admixture and dialysis. The company is committed to eliminating preventable treatment errors and enhancing patient, clinician and environmental safety. B. Braun Medical is headquartered in Bethlehem, Pennsylvania and is part of the B. Braun Group of Companies in the U.S., which includes B. Braun Interventional Systems, Aesculap® and CAPS®.
Globally, the B. Braun Group of Companies employs more than 64,000 employees in 64 countries. Guided by its Sharing Expertise® philosophy, B. Braun continuously exchanges knowledge with customers, partners and clinicians to address the critical issues of improving care and lowering costs. To learn more about B. Braun Medical, explore our website.

Source: FDA
B. Braun Issues Voluntary Nationwide Recall of 0.9% Sodium Chloride for Injection USP 1000 mL in E3 Containers Due to the Potential for Particulate Matter and Leakage
BETHLEHEM, PA - August 8, 2024 – B. Braun Medical Inc. (B. Braun), is voluntarily recalling two (2) lots of 0.9% Sodium Chloride for Injection USP 1000 mL in E3 containers within the United States to the consumer level. The voluntary recall has been initiated due to the potential for particulate matter and fluid leakage of the respective containers.
The affected batches were inadvertently released to the market prior to the completion of the required acceptance activities for embedded particulate matter which may result in leakage. To date, there have been no customer complaints received and there have been no reports of serious injury or death associated with this issue.
Risk Statement: There is a reasonable probability of embolic phenomena such as stroke or ischemia/infarct to other organs and possible infection if these particulates are not sterile that could lead to permanent damage or impairment of body function which could be life-threatening.
|
Product Catalog Number |
NDC Number |
Product Description |
Lot Number |
Distribution Range |
Expiration Date |
Region Distributed |
|---|---|---|---|---|---|---|
| E8000 | 0264-7800-09 | NaCl Inj. 0.9% 1000mL – E8000 |
J2L763, J2L764 |
01.Feb.2024 – 28.Feb.2024 | 31.Mar.2025 | United States |
This intravenous solution is indicated for use in adults and pediatric patients as a source of electrolytes and water for hydration. 0.9% Sodium Chloride for Injection USP in E3 is indicated for extracellular fluid replacement, treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. 0.9% Sodium Chloride Injection USP is also indicated for use as a priming solution in hemodialysis procedures and may be used to initiate and terminate blood transfusions without hemolyzing red blood cells. Sodium Chloride Injection USP is also indicated as a pharmaceutic aid and diluent for the infusion of compatible drug additives. Product was distributed Nationwide within the United States to domestic distributors.
B. Braun has notified its distributors and customers by an official recall notice sent via certified registered mail and has arranged for return of all recalled products. Facilities and distributors that have product which is being recalled should discontinue use immediately and contact the B. Braun Medical Inc. Customer Support Department at 800-227-2862 Monday through Friday, 8 a.m. – 6 p.m. EST to arrange for product return.
Adverse reactions or quality problems experienced with this product, or questions about this recall may be reported to B. Braun’s Postmarket Surveillance Department by calling 1-833-425-1464.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About B. Braun
B. Braun Medical Inc. (B. Braun) is a leader in smart infusion therapy and safe and effective pharmacy products, patient and provider safety, and sustainable health solutions. Our purpose is to help providers constantly improve patient satisfaction and outcomes. With products and services created to help healthcare professionals focus on what matters most—their patients—we’re uniquely positioned to help health systems succeed now and in the future. B. Braun is headquartered in Bethlehem, Pennsylvania and is part of the B. Braun Group of Companies in the U.S., which includes B. Braun Interventional Systems, Aesculap® and CAPS®. The company employs 8,500 people at over 30 locations across North America. Globally, the B. Braun Group of Companies employs more than 64,000 employees in 64 countries. Guided by its Sharing Expertise® philosophy, B. Braun continuously exchanges knowledge with customers, partners and clinicians to address the critical issues of improving care and lowering costs.
Source: FDA
Medline Industries Recalls Hudson RCI Addipak Unit Dose Vial, 0.9% Full Normal Saline Solution Due to Being Non-Sterile
Recalled Product
- Product Names: Hudson RCI Addipak Unit Dose Vial, 0.9% Full Normal Saline Solution
- Product Codes: See Recall Database Entry
- Lot number: 3B085
- Distribution Dates: April 20, 2023 to July 14, 2023
- Devices Recalled in the U.S.: 18,000
- Date Initiated by Firm: July 24, 2023
Device Use
The Hudson RCI Addipak Unit Dose Vials is a pack of single use 0.9% Full Normal Saline Solution used during treatments such as inhalation and irrigation therapy. It may be used with a non-ventilatory nebulizer to clean the lungs or for tracheal irrigation. For inhalation therapy, the saline solution restores moisture to the lungs, relieves congestion caused by colds or allergies, or dilutes bronchodilator (rescue inhaler) solutions that require dilution. For irrigation therapy, the saline solution is used for wound cleansing and flushing.
Reason for Recall
Medline Industries is recalling the Hudson RCI Addipak Unit Dose Vial, 0.9% Full Normal Saline Solution (Lot 3B085) due to being non-sterile. The affected lot passed sterility testing, however, another lot within the same cleaning cycle (Lot 3B087) failed sterility testing, exposing the affected lot to potential contamination.
The use of non-sterile saline may cause serious adverse health consequences including difficulty breathing, general discomfort, nausea, wheezing, and infection. Infection can lead to life-threatening sepsis, and death.
There have been no reports of injuries or death associated with this issue.
Who May be Affected
- People who use Hudson RCI Addipak Unit Dose Vial, 0.9% Full Normal Saline Solution for inhalation and irrigation therapy.
- Health care personnel who prescribe or provide inhalation or irrigation therapy.
What to Do
On August 4, 2023, Medline Industries sent all affected customers a recall letter.
The letter requested customers to:
- Check stock immediately for the affected item number and the affected lot numbers listed on the recall portal.
- Use the link and the information below to complete your response form. List the quantity of affected product you have in inventory on the form. Even if you do not have any affected product in inventory, please complete and submit the form.
- The login for completing the response form is:
Website link: https://recalls.medline.com
Recall Reference #: R-23-122
Recall Code: - Destroy affected product upon completion of the form. Your account will receive credit once the response form is submitted.
The letter requested distributors to:
- Notify the FDA of this recall communication if you have resold or transferred this product to another company or individual. This is required by law.
- Instruct customers to document and destroy any affected product. Include your customers quantities on your response form.
Contact Information
Customers in the U.S. with questions about this recall should contact the Medline Industries Recall Department at 866-359-1704.
Additional Resources:
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.
Source: FDA
Letter to Health Care Personnel - Prefilled Saline Flush Syringe Conservation Strategies
March 21, 2022 -- The U.S. Food and Drug Administration (FDA) is aware the United States is experiencing interruptions in the supply of prefilled 0.9% sodium chloride (saline) intravenous (IV) lock/ flush syringes. Prefilled 0.9% sodium chloride IV lock/ flush syringes are in shortage because of an increase in demand during the COVID-19 public health emergency, as well as recent vendor supply chain challenges, including the permanent discontinuance of certain prefilled saline lock/ flush syringes.
Recommendations
The FDA recommends health care personnel use prefilled 0.9% sodium chloride lock/ flush syringes, as your supply allows. When prefilled 0.9% sodium chloride lock/ flush syringes are not available, consider the following recommendations, including conservation strategies, to maintain the quality and safety of patient care:
- Use preservative-free, sterile 0.9% sodium chloride single dose vials if prefilled sterile 0.9% sodium chloride syringes are unavailable.
- Use heparin lock flush syringes, typically used to flush an IV catheter to help prevent blockage within the catheter after receiving an IV infusion, if medically appropriate and in accordance with your facility’s policy, unless contraindicated in the manufacturer’s labeling.
- Do not use expired prefilled saline flush syringes because they may have decreased volume, degraded ingredients, or lack sterility that may compromise the device’s performance and increase patient risk.
- Do not use prefilled saline flush syringes that are not FDA-cleared flush syringes.
- Contact the FDA at deviceshortages@fda.hhs.gov as well as your group purchasing organization (GPO), local product representative, distributor, or account manager if the conservation strategies are not adequate to maintain sufficient supply.
- Consider recommendations from the FDA as well as relevant professional organizations for other strategies that might be appropriate for your organization.
Background
Prefilled 0.9% sodium chloride intravenous lock/ flush syringes are single use syringes filled with sterile 0.9% sodium chloride (saline) solution, which may come in different volumes. A prefilled 0.9% sodium chloride intravenous lock/flush syringe is used to help prevent vascular access systems from becoming blocked and to help remove any medication that may be left at the catheter site.
FDA Actions
On March 21, 2022, the FDA added prefilled 0.9% sodium chloride IV saline flush syringes (product code NGT - Saline, Vascular Access Flush) to the medical device shortage list and device discontinuance list. The device shortage list reflects the types of devices the FDA determined to be in shortage. The FDA will continue to update the list as needed. The FDA also carefully reviews each notification under section 506J of the Federal Food, Drug, and Cosmetic Act received and uses this information, along with any additional details about the supply and demand of a device, to determine whether a device is in shortage.
On January 14, 2022, the FDA updated the table of device types and corresponding product codes identified as devices that FDA believes are critical to the public health during the COVID-19 pandemic under section 506J(a)(1) of the FD&C Act to include prefilled saline flush syringes (product code NGT).
The FDA lists FDA-cleared prefilled saline flush syringes in the FDA’s 510(k) Premarket Notification database under the product code NGT (Saline, Vascular Flush).
The FDA is working with manufacturers to help mitigate the shortage. The FDA continues to monitor the situation to help ensure prefilled 0.9% sodium chloride IV lock/ flush syringes are available for patients where intravenous infusions are medically necessary. The FDA will inform the public if significant new information becomes available.
Reporting Problems to the FDA
The FDA encourages health care personnel to report all adverse events or suspected adverse events experienced with any prefilled saline flush syringes.
- Voluntary reports can be submitted through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
- Device manufacturers and user facilities must comply with the applicable Medical Device Reporting (MDR) regulations.
- Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices.
In addition, the FDA would like to hear from health care personnel who have trouble obtaining devices, as well as from other stakeholders who may help mitigate potential shortages. You may email the FDA at deviceshortages@fda.hhs.gov. Note that pursuant to section 506J manufacturers must notify the FDA of an interruption or permanent discontinuance likely to lead in a meaningful disruption in the supply of these devices.
Contact Information
If you have questions about this letter, contact the FDA about a medical device supply chain issue.
Source: FDA
B. Braun Medical Inc. Issues Voluntary Nationwide Recall of 0.9% Sodium Chloride for Injection USP 250ML in Excel Due to Fluid Leakage or Low Fill Volume
March 3, 2022 -- B. Braun Medical Inc. (B. Braun) is voluntarily recalling five (5) lots of 0.9% Sodium Chloride for Injection USP 250ML in Excel within the United States to the hospital/user level. The voluntary recall has been initiated due to fluid leakage or low fill volume of the respective containers.
The biggest risk with a slow leak in any intravenous solution preparation is a break in sterility which poses a risk for the patient being exposed to a bacterial or fungal infection. There is a remote probability this could lead to bloodstream infection. B. Braun has not received any reports of adverse events related to this recall.
0.9% Sodium Chloride for Injection USP in Excel is indicated for extracellular fluid replacement, treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. 0.9% Sodium Chloride Injection USP in Excel is also indicated for use as a priming solution in hemodialysis procedures and may be used to initiate and terminate blood transfusions without hemolyzing red blood cells.
| Product Catalog Number: | Lot Number: | NDC: | Product Description: | Distribution Date Range: | Expiration Date: | Region Distributed: |
|---|---|---|---|---|---|---|
| L8002 | ||||||
| J1E086 | 0264-7800-20 | 0.9% NACL INJ USP 250ML | 15JUN2021 – 22JUL2021 | 31-May-23 | United States | |
| J1E204 | 0264-7800-20 | 0.9% NACL INJ USP 250ML | 17JUN2021 – 21JUL2021 | 31-May-23 | United States | |
| J1E213 | 0264-7800-20 | 0.9% NACL INJ USP 250ML | 02JUN2021 – 28JUN2021 | 31-May-23 | United States | |
| J1H137 | 0264-7800-20 | 0.9% NACL INJ USP 250ML | 14JUL2021 – 20OCT2021 | 30-Jun-23 | United States | |
| J1H138 | 0264-7800-20 | 0.9% NACL INJ USP 250ML | 14JUL2021 – 29OCT2021 | 30-Jun-23 | United States |
Product was distributed Nationwide within the United States to domestic distributors.
B. Braun is notifying its distributors and customers by an official recall notice sent via certified registered mail and is arranging for return of all recalled products. Facilities and distributors that have product which is being recalled should discontinue use immediately and contact the B. Braun Medical Inc. Customer Support Department at 800-227-2862 Monday through Friday, 8 a.m. – 6 p.m. EST to arrange for product return.
Facilities with questions regarding this recall can contact B. Braun by phone at 800-227-2862 Monday through Friday, 8 a.m. – 6 p.m. EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: FDA
Enovachem Pharmaceuticals Issues Voluntary Nationwide Recall of Dyural-40 and Dyural-80 Convenience Kits Containing Recalled Sodium Chloride Injection, USP, 0.9% Due to Latex Hazard
Torrance, CA, Asclemed USA Inc is voluntarily recalling 20 lots of Dyural-40 and 61 lots of Dyural-80, to the user level. The products include recalled Sodium Chloride, USP, 0.9% manufactured by Fresenius Kabi, which has been recalled due to product labeling incorrectly stating stoppers do not contain latex.
For the population most at risk, those with a severe allergic reaction to latex, there is probability of an anaphylactic reaction, and this could result in hospitalization or death. To date, Asclemed USA Inc has not received any reports of adverse events related to this recall.
The products are Dyural-40 convenience kits packaged in plastic trays and Dyural-80 convenience kits packaged in plastic trays, containing Sodium Chloride, USP, 0.9% by Fresenius Kabi.
The affected Dyural-40 lots include the following:
| LOT | EXP | LOT | EXP | LOT | EXP | LOT | EXP |
|---|---|---|---|---|---|---|---|
| 051518X5 | 1/31/2019 | 072518X3 | 5/31/2019 | 090518X4 | 6/30/2019 | 092418X1 | 8/31/2019 |
| 051618X1 | 1/31/2019 | 072718X1 | 5/31/2019 | 091818X2 | 1/31/2019 | 092818X4 | 8/31/2019 |
| 052318X4 | 5/1/2019 | 080318X2 | 5/31/2019 | 091818X3 | 7/31/2019 | 101018X3 | 8/31/2019 |
| 052318X5 | 5/31/2019 | 082318X4 | 6/30/2019 | 091818X4 | 6/30/2019 | 101018X5 | 8/31/2019 |
| 062818X1 | 5/31/2019 | 083118X1 | 6/30/2019 | 091818X5 | 8/31/2019 | 102418X5 | 9/30/2019 |
The affected Dyural-80 lots include the following:
| LOT | EXP | LOT | EXP | LOT | EXP | LOT | EXP |
|---|---|---|---|---|---|---|---|
| 050918X1 | 12/31/2018 | 062718X1 | 6/30/2019 | 080718X7 | 6/30/2019 | 100518X6 | 7/31/2019 |
| 051518X4 | 5/31/2019 | 062718X2 | 6/30/2019 | 080918X3 | 6/30/2019 | 101118X3 | 7/31/2019 |
| 051618X10 | 12/31/2018 | 062818X3 | 6/30/2019 | 083018X2 | 6/30/2019 | 101518X2 | 7/31/2019 |
| 051818X4 | 12/31/2018 | 062818X4 | 6/30/2019 | 083118X2 | 6/30/2019 | 101618X7 | 7/31/2019 |
| 051818X5 | 5/31/2019 | 070918X1 | 6/30/2019 | 083118X5 | 6/30/2019 | 101618X8 | 7/31/2019 |
| 052118X4 | 5/31/2019 | 071018X5 | 6/30/2019 | 090518X5 | 6/30/2019 | 101818X3 | 7/31/2019 |
| 052118X5 | 5/31/2019 | 071118X4 | 6/30/2019 | 090518X6 | 7/31/2019 | 101918X1 | 7/31/2019 |
| 052918X7 | 5/31/2019 | 071118X5 | 6/30/2019 | 090718X2 | 7/31/2019 | 102318X1 | 7/31/2019 |
| 061118X8 | 5/1/2019 | 071718X2 | 2/28/2019 | 090718X3 | 7/31/2019 | 103118X1 | 7/31/2019 |
| 061118X9 | 5/31/2019 | 072018X6 | 6/30/2019 | 090718X5 | 7/31/2019 | 103118X2 | 7/31/2019 |
| 061118X10 | 5/31/2019 | 072418X3 | 6/30/2019 | 091118X7 | 7/31/2019 | 103118X3 | 9/30/2019 |
| 061418X2 | 5/31/2019 | 072418X4 | 6/30/2019 | 091318X5 | 7/31/2019 | 110618X1 | 9/30/2019 |
| 061518X1 | 5/31/2019 | 072518X2 | 6/30/2019 | 091918X1 | 7/31/2019 | 110818X1 | 9/30/2019 |
| 061518X2 | 6/30/2019 | 073018X4 | 6/30/2019 | 092718X1 | 7/31/2019 | ||
| 061918X2 | 6/30/2019 | 073018X8 | 6/30/2019 | 092718X2 | 7/31/2019 | ||
| 062518X2 | 6/30/2019 | 080218X3 | 6/30/2019 | 092818X3 | 7/31/2019 |
The products can be identified by lot and expiration stamped on the front of each convenience kit. Product was distributed Nationwide to distributors and physicians.
Asclemed USA Inc is notifying its distributors and customers by email and is arranging for return of all recalled products. Distributors and physicians that have Dyural-40 or Dyural-80 which is being recalled should stop using them and return to place of purchase.
Consumers with questions regarding this recall can contact Asclemed USA Inc by calling (310) 320-0100 ext. 120 Monday through Friday from 7:30am to 4:00pm PST or emailing christinah@enovachem.us.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfml
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Fresenius Kabi Issues Voluntary Nationwide Recall of Sodium Chloride Injection, USP, 0.9% Due to Product Labeling Incorrectly Stating Stoppers Do Not Contain Latex
Fresenius Kabi USA is voluntarily recalling 163 lots of Sodium Chloride Injection, USP, 0.9%, 10 mL fill in a 10 mL vial and Sodium Chloride Injection, USP, 0.9%, 20 mL fill in a 20 mL vial to the user level. The product insert states that stoppers for both the 10mL and the 20mL vials do not contain natural rubber latex; the tray label for the two vial sizes and the vial label for the 20mL vial also state that the stoppers do not contain latex. The product is being recalled because the stoppers contain natural rubber latex.
For the population most at risk, those with a severe allergic reaction to latex, there is probability of an anaphylactic reaction, and this could result in hospitalization or death. To date, Fresenius Kabi USA has not received any reports of adverse events related to this recall.
Sodium Chloride Injection, USP, 0.9% is indicated for diluting or dissolving drugs for intramuscular, intravenous or subcutaneous injection according to instructions of the manufacturer of the drug to be administered. It is also indicated for use in flushing of intravenous catheters. The product is packaged as Sodium Chloride Injection, USP 0.9%, 10mL fill in a 10mL vial; Sodium Chloride Injection, USP 0.9% 20mL fill in a 20mL vial; both size vials are packaged in a 25-unit tray. See the tables below for a full list of the affected lots including lot numbers and expiration dates.
Fresenius Kabi USA is notifying its distributors and customers by letter and is arranging for return of the recalled product. If health care facilities have any of the affected lots, they are to immediately discontinue distributing, dispensing or using the lots and return all units to Fresenius Kabi. Distributors are instructed to immediately notify their customers that have been shipped, or may have been shipped, the product involved in this recall.
Consumers with questions regarding this recall can contact Fresenius Kabi USA Quality Assurance at 1-866-716-2459, Monday through Friday, during the hours of 8:00 a.m. to 5:00 p.m. Central Standard Time. Consumers should contact their physician or health care provider if they have experienced any problems that may be related to receiving this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to Fresenius Kabi Medical Affairs or Vigilance departments at 1-800-551-7176, Monday through Friday, during the hours of 8:00 a.m. to 5:00 p.m. Central Standard Time, or send an e-mail to either productcomplaint.USA@fresenius-kabi.com or adverse.events.USA@fresenius-kabi.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax.
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. For more information about Fresenius Kabi worldwide, please visit www.fresenius-kabi.com.
| Product Name /Product size | Unit of Sale NDC Number | Unit of Use NDC Number | Product Code | Batch Number | Expiration Date |
|---|---|---|---|---|---|
| Sodium Chloride Injection, USP, 0.9%, 10 mL fill in a 10 mL vial | 63323-186-10 | 63323-186-01 | 918610 | 6013112 | 11/2018 |
| 6013113 | 11/2018 | ||||
| 6013114 | 11/2018 | ||||
| 6013180 | 11/2018 | ||||
| 6013181 | 11/2018 | ||||
| 6013182 | 11/2018 | ||||
| 6013237 | 01/2019 | ||||
| 6013238 | 01/2019 | ||||
| 6013239 | 01/2019 | ||||
| 6013468 | 02/2019 | ||||
| 6013512 | 02/2019 | ||||
| 6013513 | 02/2019 | ||||
| 6013551 | 02/2019 | ||||
| 6013552 | 02/2019 | ||||
| 6013553 | 02/2019 | ||||
| 6013607 | 02/2019 | ||||
| 6013608 | 02/2019 | ||||
| 6013610 | 02/2019 | ||||
| 6013627 | 03/2019 | ||||
| 6013678 | 03/2019 | ||||
| 6013679 | 03/2019 | ||||
| 6013822 | 03/2019 | ||||
| 6013823 | 03/2019 | ||||
| 6013824 | 03/2019 | ||||
| 6013924 | 04/2019 | ||||
| 6013925 | 04/2019 | ||||
| 6013926 | 04/2019 | ||||
| 6014003 | 05/2019 | ||||
| 6014004 | 05/2019 | ||||
| 6014005 | 05/2019 | ||||
| Sodium Chloride Injection, USP, 0.9%, 10 mL fill in a 10 mL vial | 63323-186-10 | 63323-186-01 | 918610 | 6014260 | 05/2019 |
| 6014301 | 05/2019 | ||||
| 6014302 | 05/2019 | ||||
| 6014303 | 06/2019 | ||||
| 6014304 | 06/2019 | ||||
| 6014305 | 06/2019 | ||||
| 6014306 | 06/2019 | ||||
| 6014307 | 06/2019 | ||||
| 6014384 | 06/2019 | ||||
| 6014404 | 06/2019 | ||||
| 6014405 | 06/2019 | ||||
| 6014453 | 06/2019 | ||||
| 6014454 | 06/2019 | ||||
| 6014455 | 06/2019 | ||||
| 6014479 | 06/2019 | ||||
| 6014557 | 07/2019 | ||||
| 6014558 | 07/2019 | ||||
| 6014606 | 07/2019 | ||||
| 6014649 | 08/2019 | ||||
| 6014650 | 08/2019 | ||||
| 6014704 | 08/2019 | ||||
| 6014766 | 08/2019 | ||||
| 6014767 | 08/2019 | ||||
| 6014768 | 08/2019 | ||||
| 6014841 | 08/2019 | ||||
| 6014842 | 08/2019 | ||||
| 6014843 | 08/2019 | ||||
| 6014861 | 08/2019 | ||||
| 6014862 | 08/2019 | ||||
| 6014863 | 08/2019 | ||||
| 6015049 | 09/2019 | ||||
| 6015050 | 09/2019 | ||||
| 6015088 | 09/2019 | ||||
| Sodium Chloride Injection, USP, 0.9%, 10 mL fill in a 10 mL vial | 63323-186-10 | 63323-186-01 | 918610 | 6015118 | 10/2019 |
| 6015127 | 10/2019 | ||||
| 6015128 | 10/2019 | ||||
| 6015186 | 10/2019 | ||||
| 6015187 | 10/2019 | ||||
| 6015188 | 10/2019 | ||||
| 6015233 | 10/2019 | ||||
| 6015234 | 10/2019 | ||||
| 6015235 | 10/2019 | ||||
| 6015285 | 11/2019 | ||||
| 6015286 | 11/2019 | ||||
| 6015287 | 11/2019 | ||||
| 6015408 | 11/2019 | ||||
| 6015409 | 11/2019 | ||||
| 6015410 | 11/2019 | ||||
| 6015452 | 11/2019 | ||||
| 6015453 | 11/2019 | ||||
| 6015454 | 11/2019 | ||||
| 6015572 | 11/2019 | ||||
| 6015573 | 12/2019 | ||||
| 6015574 | 12/2019 | ||||
| 6015616 | 12/2019 | ||||
| 6015617 | 12/2019 | ||||
| 6015618 | 12/2019 | ||||
| 6015922 | 01/2020 | ||||
| 6015923 | 01/2020 | ||||
| 6015924 | 01/2020 | ||||
| 6016002 | 02/2020 | ||||
| 6016003 | 02/2020 | ||||
| 6016004 | 02/2020 | ||||
| 6016077 | 02/2020 | ||||
| 6016104 | 02/2020 | ||||
| 6016208 | 02/2020 | ||||
| Sodium Chloride Injection, USP, 0.9%, 10 mL fill in a 10 mL vial | 63323-186-10 | 63323-186-01 | 918610 | 6016209 | 02/2020 |
| 6016210 | 02/2020 | ||||
| 6016258 | 02/2020 | ||||
| 6016259 | 02/2020 | ||||
| 6016260 | 02/2020 | ||||
| 6016261 | 02/2020 | ||||
| 6016262 | 03/2020 | ||||
| 6016263 | 03/2020 | ||||
| 6016264 | 03/2020 | ||||
| 6016323 | 03/2020 | ||||
| 6016324 | 03/2020 | ||||
| 6016325 | 03/2020 | ||||
| 6016383 | 03/2020 | ||||
| 6016384 | 03/2020 | ||||
| 6016385 | 03/2020 | ||||
| 6016386 | 03/2020 | ||||
| 6016387 | 03/2020 | ||||
| 6016388 | 03/2020 | ||||
| 6016389 | 03/2020 | ||||
| 6016584 | 04/2020 | ||||
| 6016585 | 04/2020 | ||||
| 6016621 | 04/2020 | ||||
| 6016622 | 04/2020 | ||||
| 6016623 | 04/2020 | ||||
| 6016765 | 05/2020 | ||||
| 6016766 | 05/2020 | ||||
| 6016767 | 05/2020 | ||||
| 6016768 | 05/2020 | ||||
| 6016769 | 05/2020 | ||||
| 6016875 | 06/2020 | ||||
| 6016876 | 06/2020 | ||||
| 6016877 | 06/2020 | ||||
| 6016878 | 06/2020 | ||||
| Sodium Chloride Injection, USP, 0.9%, 10 mL fill in a 10 mL vial | 63323-186-10 | 63323-186-01 | 918610 | 6016879 | 06/2020 |
| 6017288 | 06/2020 | ||||
| 6017289 | 06/2020 | ||||
| 6017290 | 06/2020 | ||||
| 6017291 | 06/2020 | ||||
| 6017382 | 07/2020 | ||||
| 6017425 | 07/2020 | ||||
| 6017426 | 07/2020 | ||||
| 6017427 | 07/2020 | ||||
| 6017428 | 07/2020 | ||||
| 6017429 | 07/2020 | ||||
| 6017470 | 07/2020 | ||||
| 6017471 | 07/2020 | ||||
| 6017472 | 07/2020 | ||||
| 6017473 | 07/2020 | ||||
| 6017474 | 07/2020 | ||||
| 6017675 | 08/2020 | ||||
| 6017725 | 08/2020 | ||||
| 6017726 | 08/2020 | ||||
| Sodium Chloride Injection, USP, 0.9%, 20 mL fill in a 20 mL vial | 63323-186-20 | 63323-186-03 | 918620 | 6013062 | 11/2018 |
| 6014162 | 05/2019 | ||||
| 6014163 | 05/2019 | ||||
| 6014164 | 05/2019 | ||||
| 6014377 | 06/2019 | ||||
| 6014378 | 06/2019 | ||||
| 6014379 | 06/2019 | ||||
| 6016005 | 02/2020 | ||||
| 6016071 | 02/2020 | ||||
| 6016072 | 02/2020 | ||||
| 6016073 | 02/2020 | ||||
| 6017383 | 07/2020 | ||||
| 6017384 | 07/2020 | ||||
| 6017422 | 07/2020 | ||||
| 6017423 | 07/2020 | ||||
| 6017424 | 07/2020 |
0.9 Percent Sodium Chloride Injection by ICU Medical: Recall - Presence of Particulate Matter
[Posted 07/31/2017]
ISSUE: ICU Medical, Inc. is voluntarily recalling one lot of 0.9% Sodium Chloride Injection, USP 1000 mL to the hospital/user level due to a confirmed customer complaint of particulate matter identified as stainless steel within a single flexible container.
Injection of particulate matter could potentially lead to limited adverse events such as allergic reactions, local irritation and inflammation in organs or tissues, or other serious adverse health consequences.
BACKGROUND: 0.9% Sodium Chloride Injection, USP 1000 mL is an intravenous solution indicated for parenteral replenishment of fluid. The affected product lot was manufactured in the U.S. by Hospira, a Pfizer company, on February 1, 2016 and was distributed nationwide to Hospira customers between April 14, 2016 and February 2, 2017. The affected lot is: NDC 0409-7983-09, Lot # 61-841-FW Expires January 01, 2018 - 1000mL Single Dose Flexible Container.
RECOMMENDATION: Prior to administration, healthcare professionals, as instructed in the product labeling, should visually examine the product for particulate matter and discoloration and should discard if a defect is identified.
ICU Medical is notifying its distributors and customers of this recall by letter and is arranging for the return of all recalled products. Hospitals/distributors that have product that is being recalled should stop use/further distribution and return to place of purchase. Customers with questions regarding this recall can call ICU Medical at 1-800-441-4100 Monday through Friday between the hours of 8 a.m. and 5 p.m. Central time. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[07/28/2017 - Press Release - ICU Medical, Inc.]
Baxter IV Solutions: Recall - Potential Presence of Particulate Matter
Includes:
- 0.9% Sodium Chloride Injection, USP, 250 mL VIAFLEX Plastic Container
- 70% Dextrose Injection (2000 mL) USP
ISSUE: Baxter International Inc. announced a voluntarily recall of two lots of intravenous (IV) solutions to the hospital/end user level due to the potential presence of particulate matter. The particulate matter in each case was determined to be an insect and was identified as a result of a customer complaint.
The lots being recalled were distributed to customers and distributors in the United States between June 6, 2015 and December 16, 2015. See the Press Release for affected lot numbers.
Injecting a product containing particulate matter, in the absence of in-line filtration, may result in blockage of blood vessels, which can result in stroke, heart attack or damage to other organs such as the kidney or liver. There is also the possibility of allergic reactions, local irritation and inflammation in tissues and organs.
BACKGROUND: 0.9% Sodium Chloride Injection, USP, 250 mL VIAFLEX Plastic Container is intended for IV use as a source of water and electrolytes and may also be used as a priming solution in hemodialysis procedures. 70% Dextrose Injection (2000 mL) USP is indicated as a source of calories and water for hydration.
RECOMMENDATION: Baxter is directing customers not to use the product from the recalled lots. Recalled product should be returned to Baxter for credit by contacting Baxter Healthcare Center for Service at 1-888-229-0001, Monday through Friday, between the hours of 7 a.m. and 6 p.m., Central Time. Unaffected lots of product are available for replacement. This recall is not expected to affect current supply and product remains available for current customers.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[12/18/2015 - Press Release - Baxter]
0.9 Percent Sodium Chloride Injection, USP (AUTO-C) by Baxter International: Recall - Potential For Leaking Containers, Particulate Matter and Missing Port
ISSUE: Baxter International Inc. announced a voluntarily recall of one lot of intravenous (IV) solution to the hospital/user level due to the potential for leaking containers, particulate matter and missing port protectors. Leaking containers, particulate matter and missing port protectors could result in contamination of the solution. If not detected, this could lead to a bloodstream infection or other serious adverse health consequences. Injecting a product containing particulate matter, in the absence of in-line filtration, may result in blockage of blood vessels, which can result in stroke, heart attack or damage to other organs such as the kidney or liver. There is also the possibility of allergic reactions, local irritation and inflammation in tissues and organs.
BACKGROUND: The lot being recalled was distributed to customers and distributors nationwide between January 22, 2015 and February 12, 2015. This recall affects the following lot Number: C964601, NDC: 0338-0049-03; Expiration Date: 04/30/2016
RECOMMENDATION: Customers were notified via letter that they should not use product from the recalled lot. Recalled product should be returned to Baxter for credit by contacting Baxter Healthcare Center for Service at 1-888-229-0001, Monday through Friday, between the hours of 7:00 a.m. and 6:00 p.m., Central Time. Unaffected lots of product are available for replacement.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[07/30/2015 - Press Release - Baxter International Inc]
0.9 Percent Sodium Chloride Injection, USP, 50mL and 100mL by Baxter: Recall - Particulate Matter
ISSUE: Baxter International Inc. announced it is voluntarily recalling two lots of intravenous (IV) solutions to the hospital/user level due to the potential presence of particulate matter. The particulate matter in each case was determined to be an insect and was identified as a result of a customer complaint. The matter was identified prior to patient administration and there have been no adverse events associated with this issue reported to Baxter.
Injecting a product containing particulate matter, in the absence of in-line filtration, may result in blockage of blood vessels, which can result in stroke, heart attack or damage to other organs such as the kidney or liver. There is also the possibility of allergic reactions, local irritation and inflammation in tissues and organs.
This recall affects Lot Numbers P319921 and P327635.
BACKGROUND: The lots being recalled were distributed to customers and distributors in the United States between October 7, 2014 and July 14, 2015.
RECOMMENDATION: Baxter is directing customers not to use product from the recalled lots. Recalled product should be returned to Baxter for credit by contacting Baxter Healthcare Center for Service at 1-888-229-0001, Monday through Friday, between the hours of 7:00 a.m. and 6:00 p.m., Central Time. Unaffected lots of product are available for replacement.
Consumers with questions regarding this recall can call Baxter at 1-800-422-9837, Monday through Friday, between the hours of 8:00 a.m. and 5:00 p.m. Central Time, or e-mail Baxter at onebaxter@baxter.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[07/17/2015 - Press Release - Baxter]
Intravenous (IV) Solutions (Select Lots) by Baxter: Recall - Potential Presence of Particulate Matter
[Posted 04/10/2015]
ISSUE: Baxter International Inc. is voluntarily recalling select lots of intravenous (IV) solutions to the hospital/user level due to the potential presence of particulate matter. Intravenous administration of a solution containing sterile particulate matter may lead to adverse health consequences. The extent and severity of harm depends on the size, number, and composition of the foreign material, and patient’s underlying medical condition. In the absence of in-line filtration, these particles may cause: local vein irritation, inflammatory reaction, aggravation of preexisting infections, allergic reactions, and systemic embolization. In high-risk patients this may lead to serious adverse health consequences.
BACKGROUND: The lots being recalled were distributed to customers and distributors in the United States and Bermuda between January 14, 2015 and March 5, 2015. See the press release for a listing of affected products.
While Baxter manufacturing personnel were performing routine maintenance, particulate matter was detected and identified as material from a solution transmission system pump. There have been no adverse events or product complaints associated with this issue reported to Baxter. Baxter began the customer notification process on March 24, 2015.
RECOMMENDATION: Customers have been directed not to use products from the recalled lots. Recalled products should be returned to Baxter for credit by contacting Baxter Healthcare Center for Service at 1-888-229-0001, Monday through Friday, between the hours of 7:00 a.m. and 6:00 p.m., Central Time. Unaffected lots of product are available for replacement.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[04/09/2015 - Press Release - Baxter]
IV Solutions from Wallcur of San Diego: CDER Statement - FDA Warns Health Care Professionals Not to Inject Patients
UPDATED 04/08/2015. FDA’s laboratory analysis of Wallcur’s simulated Practi-0.9% sodium chloride IV is now complete. FDA sampled 11 of Wallcur’s simulated saline solution bags and identified large amounts of endotoxin and significant bacterial contamination in the samples.
These include bacteria (e.g., Bacillus spp., Brevundimonas sp., Pseudomonas spp., Rhizobium radiobacter, Sphingomonas koreensis, Sphingomonas trueperi, Sphingobium sp.). It is possible that additional bacteria are present in other bags that were not included in this analysis.
FDA is aware of more than 40 individuals who received infusions of the simulated Practi-0.9% sodium chloride IV products; 26 of whom reported adverse events that ranged from flu-like symptoms to sepsis, a potentially life-threatening complication of an infection. Of those 26 individuals, 2 deaths and 11 hospitalizations were reported.
FDA is reiterating its previous recommendations that ask health care professionals and consumers to do the following:
- Visually inspect all current IV solution bags. Ensure none of the bags are labeled “Wallcur,” “Practi-0.9% sodium chloride,” or “For clinical training purposes only”;
- Consider reviewing clinic procedures and make sure there are procedures in place to visually inspect all future shipments of IV products to ensure they are appropriate for patient use;
- Seek medical attention if you were given a simulated Practi-0.9% sodium chloride product and you experience the symptoms described above;
- Report any suspected adverse events associated with accidental or intentional exposure to simulated products to FDA’s MedWatch program online or at 1-800-332-1088.
FDA has been working closely with Wallcur to make changes to its labeling and distribution practices to prevent future occurrences. FDA also has been working with the simulation medical products industry to highlight the risks associated with the incorrect use of these products.
UPDATED 01/10/2015. Wallcur’s Practi-IV solutions bags are recalled.
AUDIENCE: Risk Manager, Health Professionals, Pharmacy
ISSUE: The U.S. Food and Drug Administration is alerting health care professionals not to use Wallcur, LLC, simulated intravenous (IV) products in human or animal patients. These products are for training purposes only. There have been reports of serious adverse events associated with the use of certain of these products – i.e., Practi IV Solution Bags.
BACKGROUND: FDA has become aware that some Wallcur training IV products have been distributed to health care facilities and administered to patients. FDA will continue to investigate and monitor this issue. The agency is also working with the Centers for Disease Control and Prevention to inform health care professionals and state health departments.
RECOMMENDATION: Before administering IV solutions to patients, health care providers should carefully check the labels to ensure that the products are not training products, such as Practi IV Solution Bags marketed by Wallcur. Wallcur’s training products, which may bear the words “for clinical simulation,” are not to be administered to patients.
If you suspect that any Wallcur training IV products may have been administered to a patient, whether or not the incident has resulted in an adverse event, please report the incident to FDA’s MedWatch Adverse Event Reporting program by:
- Complete and submit the report online at www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
[01/10/2015 - Press Release - Wallcur]
[12/30/2014 - CDER Statement- FDA]
0.9 Percent Sodium Chloride Injection, USP, 250 mL VisIV Container by Hospira: Recall - Particulate Matter
ISSUE: Hospira announced a voluntary nationwide recall of one lot of 0.9% Sodium Chloride Injection, USP, 250 mL VisIV flex container (NDC 0409-7983-25, Lot 45-110-C6, Expiry 1MAR2016) to the user level due to one confirmed customer report of particulate in a single unit. The foreign particle was confirmed by Hospira as human hair free-floating within the solution. To date, Hospira has not received reports of any adverse events associated with this issue for this lot.
Injected particulate material may result in localized inflammation, phlebitis, allergic reaction, granuloma formation or microembolic effects (IV only) and/or low-level allergic response. Capillaries which may be as small as the size of a red blood cell, approximately seven microns in diameter, may become occluded. Patients with preexisting condition of trauma or other medical condition that adversely affects the microvascular blood supply are at an increased risk.
BACKGROUND: This lot was distributed nationwide from December 2014 through January 2015. Hospira has initiated an investigation to determine the root cause and corrective and preventive actions.
RECOMMENDATION: Anyone with an existing inventory of the recalled lot should stop use and distribution and quarantine the product immediately. Customers should notify all users in their facility. Customers who have further distributed the recalled product should notify any accounts or additional locations which may have received the recalled product and instruct them if they have redistributed the product to notify their accounts, locations or facilities to the consumer level. Hospira will be notifying its direct customers via a recall letter and is arranging for impacted product to be returned to Stericycle in the United States. For additional assistance, call Stericycle at 1-888-714-5079 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[03/05/2015 - Press Release - Hospira]
0.9 Percent Sodium Chloride Injection, USP, 250 mL by Hospira : Recall - Particulate Matter
[Posted 01/23/2015]
ISSUE: Hospira, Inc. announced a voluntary nationwide recall of one lot of 0.9% Sodium Chloride Injection, USP, 250 mL (NDC 0409-7983-02, Lot 44-002-JT, Expiry 1AUG2016) to the user level due to one confirmed customer report of particulate in a single unit. Hospira has identified the particulate as a human hair, sealed in the bag at the additive port area. To date, Hospira has not received reports of any adverse events associated with this issue for this lot.
Injected particulate material may result in local inflammation, phlebitis, and/or low-level allergic response. Capillaries which may be as small as the size of a red blood cell, approximately seven microns in diameter, may become occluded. Patients with preexisting condition of trauma or other medical condition that adversely affects the microvascular blood supply are at an increased risk.
BACKGROUND: The affected lot was distributed nationwide from September 2014 through November 2014. Hospira has initiated an investigation to determine the root cause and corrective and preventive actions.
RECOMMENDATION: Anyone with an existing inventory of the recalled lot should stop use and distribution and quarantine the product immediately. Customers should notify all users in their facility. Customers who have further distributed the recalled product should notify any accounts or additional locations which may have received the recalled product and instruct them if they have redistributed the product to notify their accounts, locations or facilities to the consumer level. Hospira has notified its direct customers via a recall letter and is arranging for impacted product to be returned to Stericycle in the United States. For additional assistance, call Stericycle at 1-877-877-0164 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday. Hospira will provide allocation credits and make replacement product available for contracted customers.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[01/20/2015 - Press Release - Hospira, Inc]
0.9 Percent Sodium Chloride Injection USP in 100 mL MINI-BAG PLUS Container by Baxter: Recall - Particulate Matter
ISSUE: Baxter International Inc. initiated a recall in the United States of two lots of 0.9% Sodium Chloride Injection USP in 100 mL MINI-BAG PLUS Container to the hospital/user level. The recall is being initiated as a result of two complaints (one per lot) of particulate matter that was identified as a fragment of the frangible from the vial adapter. The issue was identified upon standard visual inspection prior to patient administration.
This recall affects lot numbers P317842 and P317891.
Intravenous administration of a solution containing sterile particulate matter may lead to adverse health consequences. The extent and severity of harm depends on the size, number, and composition of the foreign material, and the patient's underlying medical condition. In the absence of in-line filtration, particles may cause: local vein irritation, inflammatory reaction, aggravation of preexisting infections, allergic reactions, and systemic embolization (blockage of blood vessels, which can result in stroke, heart attack, or damage to other organs such as the kidney or liver).
BACKGROUND: 0.9% Sodium Chloride Injection USP in 100 mL MINI-BAG PLUS Container is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered drug.
RECOMMENDATION: According to the 0.9% Sodium Chloride Injection USP in 100 mL MINI-BAG PLUS Container product labeling, the product should be inspected visually for particulate matter and discoloration whenever solution and container permit.
Baxter has notified customers, who are being directed not to use product from the recalled lots. Recalled product should be returned to Baxter for credit by contacting Baxter Healthcare Center for Service at 1-888-229-0001, Monday through Friday, between the hours of 7:00 a.m. and 6:00 p.m., Central Time. Unaffected lots of product are available for replacement.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[12/11/2014 - Press Release - Baxter International Inc.]
Sodium Chloride Injection 0.9 percent USP, 100 mL Flexible Containers by Hospira: Recall - Particulate Matter
ISSUE: Hospira notified the public that last August it initiated a voluntary nationwide user-level recall of one lot of 0.9% Sodium Chloride Injection, USP, 100 mL, Flexible Container, NDC 0409-7984-23. This action was due to one confirmed customer report where four separate particulate issues were identified in four individual flexible containers. The four single particles were identified as follows: polyester fiber, nylon fiber, cotton fiber and nitrocellulose fiber, respectively. Hospira is investigating to determine the root cause.
Affected lot number is 05-201-JT (the lot number may be followed by a -01). The affected product has an expiration date of May 1, 2013, and was distributed within the United States between May 2011 and August 2011 to wholesalers/distributors, hospitals and pharmacies.
If solution containing particulate matter is used on a patient, this may result acutely in local inflammation, phlebitis, and/or generalized low-level allergic response to the particulate and/or embolize to other organs in the body. Chronically, following sequestration, granulomatous formation in the lungs is possible.
BACKGROUND: This product is used as a source of water and electrolytes. Product was distributed within the following U.S. states: Alaska, Alabama, Arizona, California, Colorado, Florida, Georgia, Hawaii, Iowa, Idaho, Illinois, Indiana, Kansas, Kentucky, Louisiana, Massachusetts, Maryland, Michigan, Missouri, Mississippi, North Carolina, North Dakota, New Jersey, New Mexico, New York, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, Tennessee, Texas, Utah, Virginia, Washington, Wisconsin, West Virginia and Wyoming.
RECOMMENDATION: Anyone with an existing inventory should stop use and distribution, quarantine the product immediately, and call Stericycle at 1-888-597-9582 between the hours of 8am to 5pm ET, Monday through Friday, to arrange for the return of the product. Replacement product from other lots is available.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[04/25/2013 - Press Release - Hospira]
Sodium Chloride Injection, 0.9 percent, 1000 mL, Flexible Container: Recall - Brass Particulates
ISSUE: Hospira, Inc. notified healthcare professional of a voluntary nationwide user-level recall of one lot of 0.9% Sodium Chloride Injection, USP, 1000 mL, Flexible Container, NDC 0409-7983-09. This action is due to one confirmed customer report where brass particulate was identified in the primary container in the form of several small grey/brown particles. The affected lot number is 25-037-JT (the lot number may be followed by a -01 or -90), with an expiration date of January 1, 2015. Hospira is investigating to determine the root cause.
The brass particulate was identified as containing copper, zinc and lead. If administered, solution containing brass particulate may result in occlusion of small blood vessels. In a worst-case scenario, copper toxicity may potentially result in hemolysis and liver toxicity, including hepatic necrosis which may be fatal.
BACKGROUND: The product is used as a source of water and electrolytes and is packaged in a 1000 mL flexible container. The affected lot was distributed nationwide between January 2013 and March 2013 to wholesalers/distributors, hospitals and pharmacies.
RECOMMENDATION: Anyone with an existing inventory should stop use and distribution, quarantine the product immediately, and call Stericycle at 1-888-480-2853 between the hours of 8am to 5pm EST, Monday through Friday, to arrange for the return of the product. Replacement product from other lots is available.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[03/29/2013 - Press Release - Hospira, Inc]
American Regent Injectable Products: Recall - Visible Particulates in Products
- Methyldopate HCL Injection, USP 5ml Single Dose Vial
- Caffeine & Sodium Benzoate Injection, USP, 250 mg/mL, 2 mL Single Dose Vial
- Ammonium Molybdate Injection, USP (Molybdenum 250mcg/10 mL) 10 mL Single Dose Vial
- Dexamethasone Sodium Phosphate Injection, 4 mg/mL, 1 mL Single Dose Vials; 5 mL and 30 mL Multiple Dose Vials
- Bacteriostatic Sodium Chloride Injection, USP, 0.9%, 30 mL Multiple Dose Vials
- Concentrated Sodium Chloride Injection, USP 23.4%, 30 mL Single Dose Vials and 100mL Pharmacy Bulk Packages
- Sodium Thiosulfate Injection USP 10%
- Potassium Phosphates Injection, USP
[UPDATED 06/07/2011] Methyldopate HCL Injection, USP 5ml Single Dose Vial recalled.
[UPDATED 05/06/2011] Caffeine & Sodium Benzoate Injection, USP, 250 mg/mL, 2 mL Single Dose Vial recalled.
[UPDATED 04/27/2011] Ammonium Molybdate Injection, USP (Molybdenum 250mcg/10 mL) 10 mL Single Dose Vial recalled.
[UPDATED 03/18/2011] Dexamethasone Sodium Phosphate Injection products recalled.
[UPDATED 03/17/2011] Concentrated Sodium Chloride Injection products recalled.
[Posted 02/05/2011]
ISSUE: Recall initiated because some vials exhibit translucent visible particles consistent with glass delamination. Potential adverse events after intravenous administration include damage to blood vessels in the lung, localized swelling, and granuloma formation.
BACKGROUND: Glass delamination can occur with high pH solutions when the surface glass from the vial separates into thin layers, resulting in glass particles with a flaky appearance.
RECOMMENDATION: Hospitals, Home Health Care Agencies, Emergency Rooms, Infusion Centers, Clinics and other healthcare facilities should not use the recalled American Regent products. Recalled products should be immediately quarantined for return. Refer to Press Releases for specific lot numbers recalled.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[06/06/2011 - Press Release, Methyldopate HCL Injection - American Regent]
[05/05/2011 - Press Release, Caffeine & Sodium Benzoate Injection - American Regent]
[04/26/2011 - Press Release, Ammonium Molybdate Injection - American Regent]
[03/16/2011 - Press Release, Dexamethasone Sodium Phosphate - American Regent]
[03/15/2011 - Press Release, Bacteriostatic Sodium Chloride - American Regent]
[03/15/2011 - Press Release, Concentrated Sodium Chloride - American Regent]
[02/04/2011 - Press Release, Sodium Thiosulfate - American Regent]
[02/03/2011 - Press Release, Potassium Phosphates - American Regent]
Previous, related product alerts:
[12/24/2010 - Dexamethasone Sodium Phosphate Injection]
[12/29/2010 - Sodium Bicarbonate Injection]
Excelsior Medical 5 ml Fill in 6 cc Prefilled Saline Flush Syringes: Recall - Potential Loss of Sterility
ISSUE: Routine internal testing conducted on this product found that some of these syringes may leak and lose sterility. This recall pertains only to syringes with the following product code numbers: E0100-50, 10056-1000, 10056-240, 14056-240, 910056-1000, and S5. Exposure to syringes with a sterility issue could result in systemic infection, which may lead to serious injury and/or death.
BACKGROUND: The Excelsior Disposable 5ml fill in 6 cc prefilled saline flush syringes are intended for the flushing of venous access devices and IV tubing.
RECOMMENDATION: Consumers who have 5ml fill in 6 cc saline pre-filled syringes manufactured by Excelsior Medical should immediately discontinue using these syringes and return them to the point of purchase.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[10/15/2010 - Press Release - Excelsior Medical]
More sodium chloride resources
- BD Normal Saline flush Consumer Information
- Normal Saline flush Consumer Information
- Syrex flush Consumer Information
- Sodium chloride flush Consumer Information
- Sodium chloride inhalation Consumer Information
- Sodium chloride oral Consumer Information
- Kruschen Salts Advanced Consumer Information
- Ocu-Disal Advanced Consumer Information
- Sodium chloride (Injection) Advanced Consumer Information
- Sterile Saline Diluent Tip-Lok Syringe Advanced Consumer Information
- Syrex Advanced Consumer Information
- Sodium Chloride AHFS DI Monograph
- Sodium Chloride 20% Injection AHFS DI Monograph
- Sodium Chloride Tablets Consumer Information
- Saline Flush ZR Consumer Information
- Sodium Chloride Injection Solution Consumer Information
- Sodium Chloride Nebulizer Solution Consumer Information
- Amphenol-40 Prescribing Information
- Bacteriostatic Sodium Chloride Prescribing Information
- Corvatrol Injection Prescribing Information
- Liquivida Hydration Kit Prescribing Information
- Nacellate Injection Prescribing Information
- Polifleks Prescribing Information
- Sodium Chloride 0.45% Injection Prescribing Information
- Sodium Chloride Inhalation Solution Prescribing Information
- Sodium Chloride Injection Prescribing Information
- Sodium Chloride Injection 0.45% Prescribing Information
- Sodium Chloride Injection 14.6% Prescribing Information
- Sodium Chloride Injection 23.4% Prescribing Information
- Sodium Chloride Injection 3% 5% Prescribing Information
- Sodium Chloride Injection Hospira Prescribing Information
- Sodium Chloride Intravenous Infusion Prescribing Information
- Sodium Chloride Irrigation Prescribing Information
- Sodium Chloride Irrigation Hospira Prescribing Information
- Sodium Chloride Mini-Bag Prescribing Information
