Priorix Dosage
Generic name: MEASLES VIRUS STRAIN SCHWARTZ ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN 2512[CCID_50] in 0.5mL, MUMPS VIRUS STRAIN RIT-4385 ATTENUATED CHICK EMBRYO FIBROBLASTS LIVE ANTIGEN 15842[CCID_50] in 0.5mL, RUBELLA VIRUS STRAIN WISTAR RA 27/3 LIVE ANTIGEN 1995[CCID_50] in 0.5mL;
Dosage form: kit
Drug class: Vaccine combinations
Medically reviewed by Drugs.com. Last updated on Apr 10, 2024.
For subcutaneous injection only.
Dose and Schedule
After reconstitution, a single dose of PRIORIX is approximately 0.5 mL.
Administer according to the following schedule:
- •
- First dose – 12 through 15 months of age
- •
- Second dose – 4 through 6 years of age
If PRIORIX is not administered according to this schedule and 2 doses of measles-, mumps- and rubella-virus vaccine are recommended for an individual, there should be a minimum of 4 weeks between the first and second dose.
PRIORIX may be administered as a second dose to individuals who have received a first dose of another measles, mumps and rubella virus-containing vaccine.
Preparation
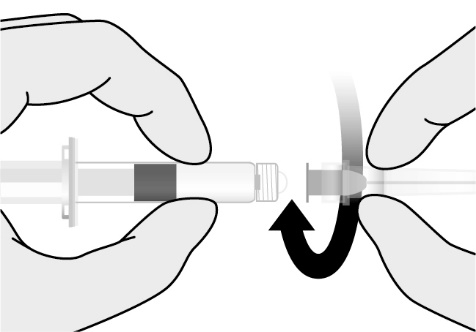
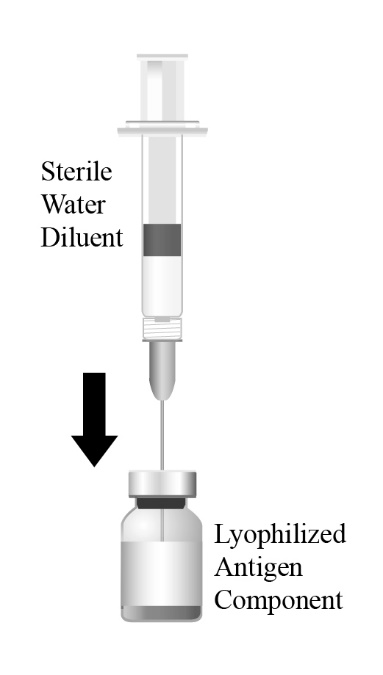
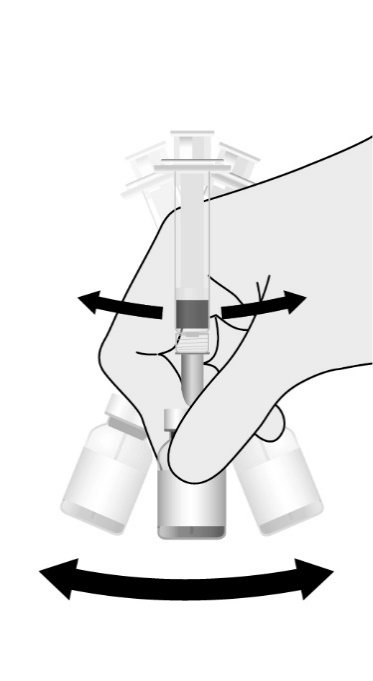
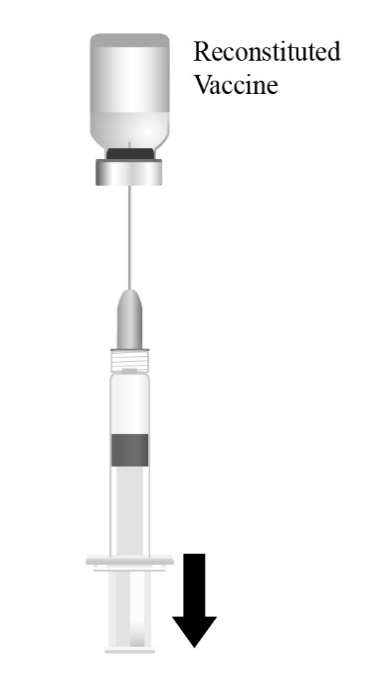
Reconstitute the Lyophilized Antigen Component, Live only with the accompanying Sterile Water Diluent Component to form PRIORIX. The reconstituted vaccine should be a clear peach- to fuchsia pink-colored suspension. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, do not administer the vaccine.
More about Priorix (measles virus vaccine / mumps virus vaccine / rubella virus vaccine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: vaccine combinations
Patient resources
Other brands
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.