Daxxify Dosage
Generic name: BOTULINUM TOXIN TYPE A 100U in 1.2mL
Dosage form: injection, powder, lyophilized, for solution
Drug class: Skeletal muscle relaxants
Medically reviewed by Drugs.com. Last updated on Jan 24, 2024.
Important Administration Instructions
The potency units of DAXXIFY for injection are specific to the preparation and test method utilized. They are not interchangeable with other preparations of botulinum toxin products, and, therefore, units of biological activity of DAXXIFY cannot be compared to, or converted into, units of any other botulinum toxin products assessed with any other specific test method.
The safe and effective use of DAXXIFY depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques.
DAXXIFY should be administered no more frequently than every three months for any indication. Consideration of the cumulative dose is necessary when treating adult patients with DAXXIFY. Physicians should be aware of whether patients are receiving treatment with other botulinum toxin products for other indications.
Reconstituted DAXXIFY is intended for intramuscular injection only.
After reconstitution, only use each DAXXIFY vial for only one injection session and for only one patient. Discard any remaining solution in vial immediately after administration.
Reconstitution instructions are provided specifically for the 50 Unit and the 100 Unit vials (Table 1; Table 2).
Recommended Dosage and Administration for Glabellar Lines
Recommended Dosage for Glabellar Lines
The total recommended dose is 40 Units per treatment session divided into five equal intramuscular injections of 8 Units each (two injections in each corrugator muscle and one injection in the procerus muscle).
Administration for Glabellar Lines
Glabellar lines arise from the contraction of the corrugator and procerus muscles. These can be identified by palpation of the glabellar muscle mass while having the patient frown maximally. Contraction of the corrugator muscles compresses the skin, creating a vertical line or lines surrounded by ridges of tensed muscle. Because the exact location, size, and activity of the muscles can vary markedly among individuals, physicians administering DAXXIFY must understand the relevant anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures and diseases. After assessment, the location of the corrugator muscle injection sites may need to be adjusted based on individual facial anatomy and the pattern of muscle contraction.
The upper eyelid margin position should be carefully examined for separation or weakness of the levator palpebrae superioris muscle. Evaluate the range of upper eyelid excursion while manually immobilizing the frontalis to assess degree of levator function and frontalis compensation.
In order to reduce the complication of ptosis, the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Ensure the injected volume/dose is accurate and administer in a steady, controlled manner.
- Do not inject DAXXIFY less than 1 centimeter above the superior orbital rim.
To inject DAXXIFY, clean the exposed portion of the stopper with an alcohol swab and aseptically withdraw at least 0.5 mL of the reconstituted solution from the vial into a sterile syringe. Replace the needle used to withdraw the product with a 30- to 33-gauge sterile needle for injection. Expel any air bubbles prior to administration.
Advance the needle through the skin into the underlying muscle while applying finger pressure on the superior medial orbital rim.
- Inject a dose of 8 Units (0.1 mL) into each of the 5 injection sites: 2 injections into medial corrugator and lateral corrugator muscles respectively, and 1 injection in the procerus muscle (Figure 1).
FIGURE 1: INJECTION SITES FOR GLABELLAR LINES 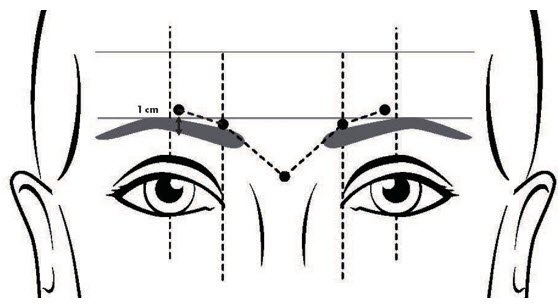
Recommended Dosage for Cervical Dystonia
The recommended dose of DAXXIFY for the treatment of cervical dystonia ranges from 125 Units to 250 Units given intramuscularly as a divided dose among affected muscles. In patients previously treated with another botulinum toxin, their past dose, response to treatment, duration of effect, and adverse event history should be taken into consideration when determining the initial DAXXIFY dose. A description of the average DAXXIFY dose and percentage of total dose injected into specific muscles in the pivotal clinical trials can be found in Section 14. Limiting the dose injected into the sternocleidomastoid muscle may reduce the occurrence of dysphagia.
Preparation and Dilution Technique
DAXXIFY is supplied in single-dose 50 Unit and 100 Unit vials. Prior to intramuscular injection, reconstitute each vial of DAXXIFY with the required amount of sterile, preservative-free 0.9% Sodium Chloride Injection, USP to obtain a reconstituted solution at the appropriate concentration described in Tables 1 and 2.
| Indication | Diluent* Added to 50 Unit Vial | Resulting Dose in Units per 0.1 mL |
|---|---|---|
|
||
| Glabellar Lines, Adults | 0.6 mL | 8 Units |
| Indication | Diluent * Added to 100 Unit Vials | Resulting Dose in Units per 0.1 mL |
|---|---|---|
|
||
| Glabellar Lines, Adults | 1.2 mL | 8 Units |
| Cervical Dystonia, Adults | 1 mL or 2 mL |
10 Units or 5 Units |
Slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Dispose of any unused diluent. Gently mix DAXXIFY with 0.9% Sodium Chloride Injection, USP by rotating the vial.
Reconstituted DAXXIFY solution is clear to slightly opalescent and colorless and free of particulate matter. Inspect visually the reconstituted DAXXIFY for particulate matter and discoloration prior to administration. Do not use if the solution is cloudy or discolored or contains flakes or particles.
Administer DAXXIFY within 72 hours after reconstitution. During this time period, store unused reconstituted DAXXIFY in a refrigerator between 2°C to 8°C (36°F to 46°F) and protected from light. Do not freeze reconstituted DAXXIFY. Dispose of any unused DAXXIFY.
Frequently asked questions
- Daxxify vs Botox: Which should you use?
- What is Daxxify, the recent FDA-alternative to Botox?
- How long does Daxxify take to work?
More about Daxxify (daxibotulinumtoxinA)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (3)
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: skeletal muscle relaxants
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Xeomin
Xeomin (incobotulinumtoxinA) is used to treat cervical dystonia, blepharospasm, upper facial lines ...
Dysport
Dysport (abobotulinumtoxinA) is used to treat cervical dystonia, glabellar lines and limb ...
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Botox Cosmetic
Botox Cosmetic is a prescription treatment for fine lines and wrinkles. It temporarily improves the ...
RimabotulinumtoxinB
RimabotulinumtoxinB is used for cervical dystonia, sialorrhea
IncobotulinumtoxinA
IncobotulinumtoxinA is used for blepharospasm, cervical dystonia, crow's feet, facial wrinkles ...
AbobotulinumtoxinA
AbobotulinumtoxinA is used for cervical dystonia, facial wrinkles, lower limb spasticity, upper ...
OnabotulinumtoxinA
OnabotulinumtoxinA information from Drugs.com, includes OnabotulinumtoxinA side effects ...
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.