The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Albon Injection 40%
This page contains information on Albon Injection 40% for veterinary use.The information provided typically includes the following:
- Albon Injection 40% Indications
- Warnings and cautions for Albon Injection 40%
- Direction and dosage information for Albon Injection 40%
Albon Injection 40%
This treatment applies to the following species: Manufacturer: Pfizer Animal Health
Manufacturer: Pfizer Animal Health
(sulfadimethoxine)
Each mL contains 400 mg of sulfadimethoxine.
Albon Injection 40% Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.Albon Injection 40% Indications For Use
Dogs and Cats: For the treatment of sulfadimethoxine-sensitive bacterial infections in dogs and cats and bacterial enteritis associated with coccidiosis in dogs. (See Section 1.) Horses: For the treatment of respiratory disease caused by Streptococcus equi (strangles). (See Section 2.) Cattle: For the treatment of bovine respiratory disease complex (shipping fever complex) and bacterial pneumonia associated with Pasteurella spp. sensitive to sulfadimethoxine; necrotic pododermatitis (foot rot) and calf diphtheria caused by Fusobacterium necrophorum (Sphaerophorus necrophorus) sensitive to sulfadimethoxine. (See Section 2.)
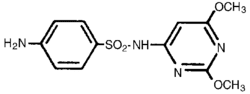
Description: Albon Injection 40% Is A Low-dosage, Rapidly Absorbed, Long-acting Sulfonamide, Effective For The Treatment Of A Wide Range Of Bacterial Infections Commonly Encountered In Dogs And Cats; The Treatment Of Respiratory Disease Of Horses, And The Treatment Of Shipping Fever Complex, Bacterial Pneumonia, Calf Diphtheria, And Foot Rot In Cattle. Sulfadimethoxine Is A White, Almost Tasteless And Odorless Compound. Chemically, It Is N1-(2,6-dimethoxy-4-pyrimidinyl) Sulfanilamide. The Structural Formula Is:
section 1-dogs And Cats
Actions: Sulfadimethoxine Has Been Demonstrated Clinically Or In The Laboratory To Be Effective Against A Variety Of Organisms, Such As Streptococci, Klebsiella, Proteus, Shigella, Staphylococci, Escherichia, And Salmonella.1,2 These Organisms Have Been Demonstrated In Respiratory, Genitourinary, Enteric, And Soft Tissue Infections Of Dogs And Cats.
the Systemic Sulfonamides Which Include Sulfadimethoxine Are Bacteriostatic Agents. Sulfonamides Competitively Inhibit Bacterial Synthesis Of Folic Acid (pteroylglutamic Acid) From Para-aminobenzoic Acid. Mammalian Cells Are Capable Of Utilizing Folic Acid In The Presence Of Sulfonamides. The Tissue Distribution Of Sulfadimethoxine, As With All Sulfonamides, Is A Function Of Plasma Levels, Degree Of Plasma Protein Binding, And Subsequent Passive Distribution In The Tissues Of The Lipid-soluble Un-ionized Form. The Relative Amounts Are Determined By Both Its Pka And By The Ph Of Each Tissue. Therefore, Levels Tend To Be Higher In Less Acid Tissue And Body Fluids Or Those Diseased Tissues Having High Concentrations Of Leucocytes.2
in The Dog, Sulfadimethoxine Is Not Acetylated As In Most Other Animals, And It Is Excreted Predominantly As The Unchanged Drug.3 Sulfadimethoxine Has A Relatively High Solubility At The Ph Normally Occurring In The Kidney, Precluding The Possibility Of Precipitation And Crystalluria. Slow Renal Excretion Results From A High Degree Of Tubular Reabsorption,4 And Plasma Protein Binding Is Very High, Providing A Blood Reservoir Of The Drug. Thus, Sulfadimethoxine Maintains Higher Blood Levels Than Most Other Long-acting Sulfonamides. Single, Comparatively Low Doses Of Albon Give Rapid And Sustained Therapeutic Blood Levels.1
to Assure Successful Sulfonamide Therapy (1) The Drug Must Be Given Early In The Course Of The Disease, And It Must Produce A High Sulfonamide Level In The Body Rapidly After Administration, (2) Therapeutically Effective Sulfonamide Levels Must Be Maintained In The Body Throughout The Treatment Period, (3) Treatment Should Continue For A Short Period Of Time After The Clinical Signs Have Disappeared, And (4) The Causative Organisms Must Be Sensitive To This Class Of Drugs.
toxicity And Safety
Data regarding acute and chronic toxicities of sulfadimethoxine indicate the drug is very safe. The LD50 in mice is greater than 2 g/kg of body weight when administered intraperitoneally and greater than 16 g/kg when administered orally. In dogs receiving massive single oral doses of 3.2 g/kg of body weight, diarrhea was the only adverse effect observed. Dogs given 160 mg/kg of body weight orally daily for 13 weeks showed no signs of toxicity.Albon Injection 40% Indications
Albon Injection 40% is indicated for the treatment of a wide range of respiratory, genitourinary tract, enteric, and soft tissue infections. For example: tonsillitis, pharyngitis, bronchitis, pneumonia, cystitis, nephritis, metritis, pyometra, pustular, dermatitis, anal gland infections, abscesses, wound infections, bacterial enteritis, canine salmonellosis, bacterial enteritis associated with coccidiosis in dogs, when caused by streptococci, staphylococci, escherichia, salmonella, klebsiella, proteus or shigella organisms sensitive to sulfadimethoxine.Limitations
Sulfadimethoxine is not effective in viral or rickettsial infections, and as with any antibacterial agent, occasional failures in therapy may occur due to resistant microorganisms. The usual precautions in sulfonamide therapy should be observed.Precautions
During treatment period, make certain that animals maintain adequate water intake. If animals show no improvement within 2 or 3 days, consult your veterinarian. Intramuscular administration is not recommended. Some animals treated by the intramuscular route exhibit signs of pain during and for a few minutes following such injections, and in dogs blood levels are lower than those obtained by intravenous treatment.Albon Injection 40% Dosage And Administration
Albon Injection 40% is recommended only for administration by the intravenous route. Usually the injectable formulation may be used to obtain effective blood levels almost immediately or to facilitate treatment of the fractious animal, and the oral formulations utilized for maintenance therapy. However, the injectable formulation may be used for the entire course of Albon therapy when indicated.Dogs and cats should receive 1 mL of Albon Injection 40% per 16 lb of body weight (55 mg/kg) as an initial dose, followed by 1/2 mL per 16 lb of body weight (27.5 mg/kg) every 24 hours thereafter. Representative weights and doses are indicated in the following table:
Each mL contains 400 mg of sulfadimethoxine.
|
Animal Weight |
Initial Dose25 Mg/lb (55 Mg/kg) |
Subsequent Daily Doses12.5 Mg/lb (27.5 Mg/kg) |
|
8 lb (3.6 kg) |
1/2 mL |
1/4 mL |
|
16 lb (7.3 kg) |
1 mL |
1/2 mL |
|
32 lb (14.5 kg) |
2 mL |
1 mL |
|
64 lb (29.1 kg) |
4 mL |
2 mL |
Length of treatment depends on the clinical response. In most cases treatment for 3-5 days is adequate. Treatment should be continued until the animal is asymptomatic for 48 hours.
Note
Store at room temperature. Should crystallization occur at cold temperatures, crystals will dissolve either by storing at room temperature for several days or by heating the vial in warm water. Crystallization and redissolution do not impair the efficacy of the product.How Supplied
Albon Injection 40% is available in 100-mL, multiple-dose vials for dogs and cats. Each mL contains 400 mg of sulfadimethoxine compounded with 20% propylene glycol, 1% benzyl alcohol, 0.1 mg disodium edetate, 1.0 mg sodium formaldehyde sulfoxylate, and pH adjusted with sodium hydroxide.Section 2-horses And Cattle
Actions
General principles of sulfonamide treatment, antibacterial spectrum of activity, and the tissue distribution of sulfadimethoxine are discussed in Section 1, Dogs and Cats, under ACTIONS. In the horse, the concentration of sulfadimethoxine has been determined to be higher in the wall of the duodenum than in any other part of the intestine. This, together with the high drug concentration in the bile, suggests enterohepatic cycling of the drug. Significant drug concentrations were also present in the cerebro-spinal fluid.5 Single, comparatively low doses of Albon give rapid and sustained therapeutic blood levels.1Toxicity And Safety
No toxic effects were noted in horses receiving up to 3 times the recommended dosage as a single injection or twice the recommended dosage for an entire course of therapy. In cattle sulfadimethoxine has been shown to be safe through extensive clinical use with other dosage forms. In addition, studies with intravenous administration of Albon Injection 40% have demonstrated that hemolysis of erythrocytes does not occur by this route of administration. Sulfadimethoxine has a high solubility at the pH normally occurring in the kidney, precluding the possibility of precipitation and crystalluria. Toxicity data in laboratory animals is discussed in Section 1, Dogs and Cats, under TOXICITY AND SAFETY.Albon Injection 40% Indications
Horses: Albon Injection 40% is indicated for the treatment of respiratory disease caused by Streptococcus equi (strangles). Cattle: Albon Injection 40% is indicated for the treatment of bovine respiratory disease complex (shipping fever complex) and bacterial pneumonia associated with Pasteurella spp. sensitive to sulfadimethoxine; necrotic pododermatitis (foot rot) and calf diphtheria caused by Fusobacterium necrophorum (Sphaerophorus necrophorus) sensitive to sulfadimethoxine.Limitations
See Section 1, Dogs and Cats, under LIMITATIONS. |
WarningsMilk taken from animals during treatment and for 60 hours (5 milkings) after the latest treatment must not be used for food. Do not administer within 5 days of slaughter. Not for use in horses intended for food.A withdrawal period has not been established for this product in preruminating calves. Do Not Use in Calves to be Processed for Veal |
 |
Precautions
During treatment period, make certain that animals maintain adequate water intake. If animals show no improvement within 2 or 3 days, reevaluate your diagnosis.Albon Injection 40% Dosage And Administration
Albon Injection 40% must be administered only by the intravenous route in horses and cattle. Horses and cattle should receive 1 mL of Albon Injection 40% per 16 lb of body weight (55 mg/kg) as an initial dose, followed by 1/2 mL per 16 lb of body weight (27.5 mg/kg) every 24 hours thereafter. Albon Boluses may be utilized for maintenance therapy in cattle. Representative weights and doses are indicated in the following table: Each mL contains 400 mg of sulfadimethoxine.
Animal Weight |
Initial Dose
|
Subsequent Daily Doses
|
|
250 lb (113.6 kg) |
15.6 mL |
7.8 mL |
|
500 lb (227.2 kg) |
31.2 mL |
15.6 mL |
|
750 lb (340.9 kg) |
46.9 mL |
23.5 mL |
|
1000 lb (454.5 kg) |
62.5 mL |
31.3 mL |
Length of treatment depends on the clinical response. In most cases treatment for 3-5 days is adequate. Treatment should be continued until the animal is asymptomatic for 48 hours.
Note: For Storage Information See Note In Section 1, Dogs And Cats, Under Dosage And Administration.
how Supplied
Albon Injection 40% is available in 100-mL, multiple-dose vials for horses and cattle. Each mL contains 400 mg of sulfadimethoxine compounded with 20% propylene glycol, 1% benzyl alcohol, 0.1 mg disodium edetate, 1.0 mg sodium formaldehyde sulfoxylate, and pH adjusted with sodium hydroxide.References
1. Data on file, Pfizer Animal Health.
2. Stowe CM: The sulfonamides. In Jones LM (ed), Veterinary Pharmacology and Therapeutics, Ames, Iowa, Iowa State University Press, chapter 33, 1995.
3. Bridges JW, Kirby MR, Walker SR, et al: Species differences in the metabolism of sulfadimethoxine. Biochem J 109:851, 1968.
4. Baggot JD: Some aspects of drug persistence in domestic animals. Res Vet Sci 11:(2)130, 1970.
5. Oh-ishi S. Tissue distribution of sulfadimethoxine and sulfamonomethoxine in horses after intravenous injection. Jap J Vet Sci 30:21, 1968.
Not for Human Use
NADA #41-245, Approved by FDA
Manufactured by: Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Missouri 64506, USA
Distributed by: Pfizer Animal Health, Exton, PA 19341, USA, Div. of Pfizer Inc, NY, NY 10017
85-8434-03
February 1999
Nac No.
36900030PFIZER ANIMAL HEALTH
235 E. 42ND ST., NEW YORK, NY, 10017
| Telephone: | 269-833-4000 | |
| Customer Service: | 800-733-5500 and 800-793-0596 | |
| Veterinary Medical Investigations & Product Support: | 800-366-5288 | |
| Technical Services (USA): | 800-366-5288 | |
| Website: | http://pfizerah.com |
 |
Every effort has been made to ensure the accuracy of the Albon Injection 40% information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27