Primene: Package Insert / Prescribing Info
Package insert / product label
Generic name: isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, alanine, aspartic acid, cysteine, glutamic acid, glycine, proline, serine, tyrosine, ornitine hydrchloride, taurine
Dosage form: injection, solution
Drug class: Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Jun 16, 2025.
Related/similar drugs
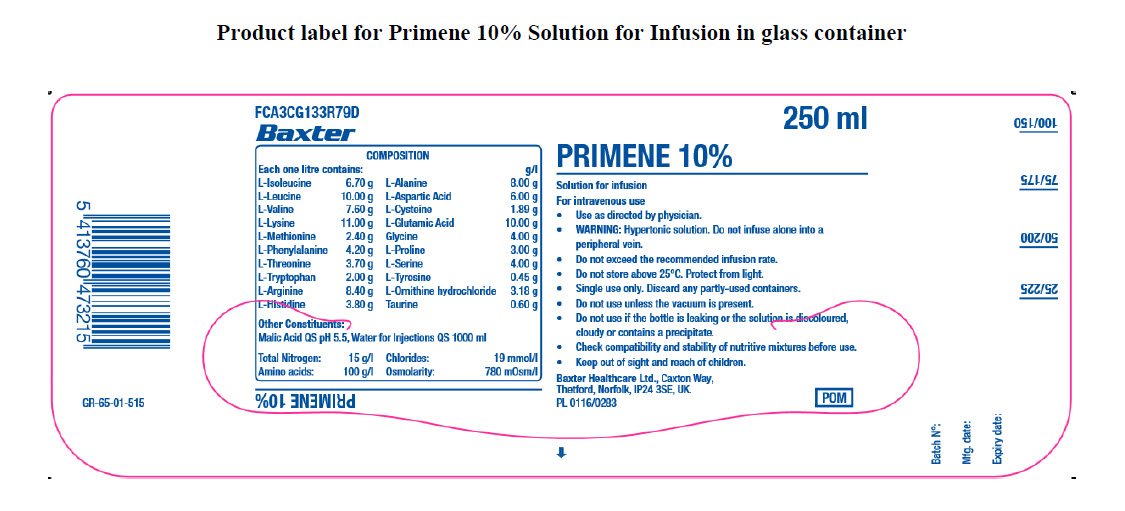
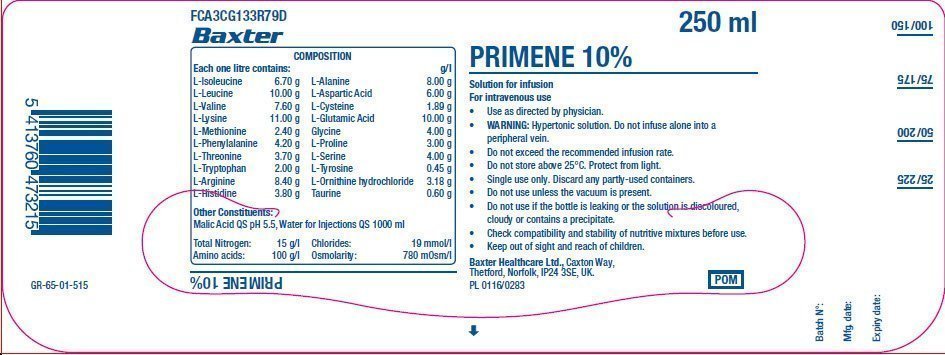
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FCA3CG133R79D
Baxter Logo
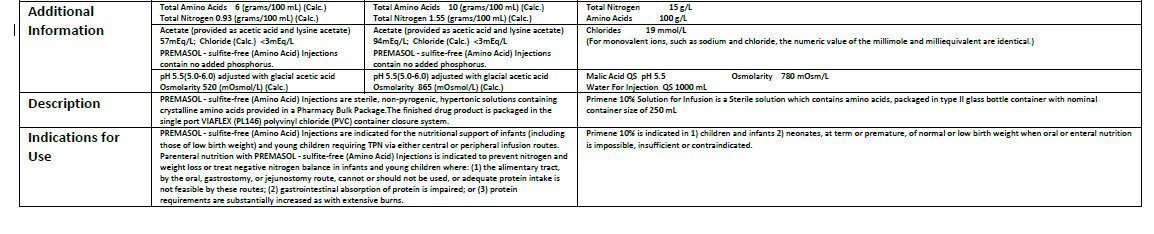
Composition
Each one litre contains: g/l
L-Isoleucine 6.70 g
L-Leucine 10.00 g
L-Valine 7.60 g
L-Lysine 11.00 g
L-Methionine 2.40 g
L-Phenylalanine 4.20 g
L-Threonine 3.70 g
L-Tryptophan 2.00 g
L-Arginine 8.40 g
L-Histidine 3.80 g
L-Alanine 8.00 g
L-Aspartic Acid 6.00 g
L-Cysteine 1.89 g
L-Glutamic Acid 10.00 g
Glycine 4.00 g
L-Proline 3.00 g
L-Serine 4.00 g
L-Tyrosine 0.45 g
L-Ornithine hydrochloride 3.18 g
Taurine 0.60 g
Other constituents:
Malic Acid QS pH 5.5, Water for Injections QS 1000 ml
Total Nitrogen: 15 g/l
Amino acids: 100 g/l
Chlorides: 19 mmol/l
Osmolarity: 780 mOsm/l
Bar Code
5413760 473215
GR-65-01-515
Primene 10%
250 ml
PRIMENE 10%
Solution for Infusion
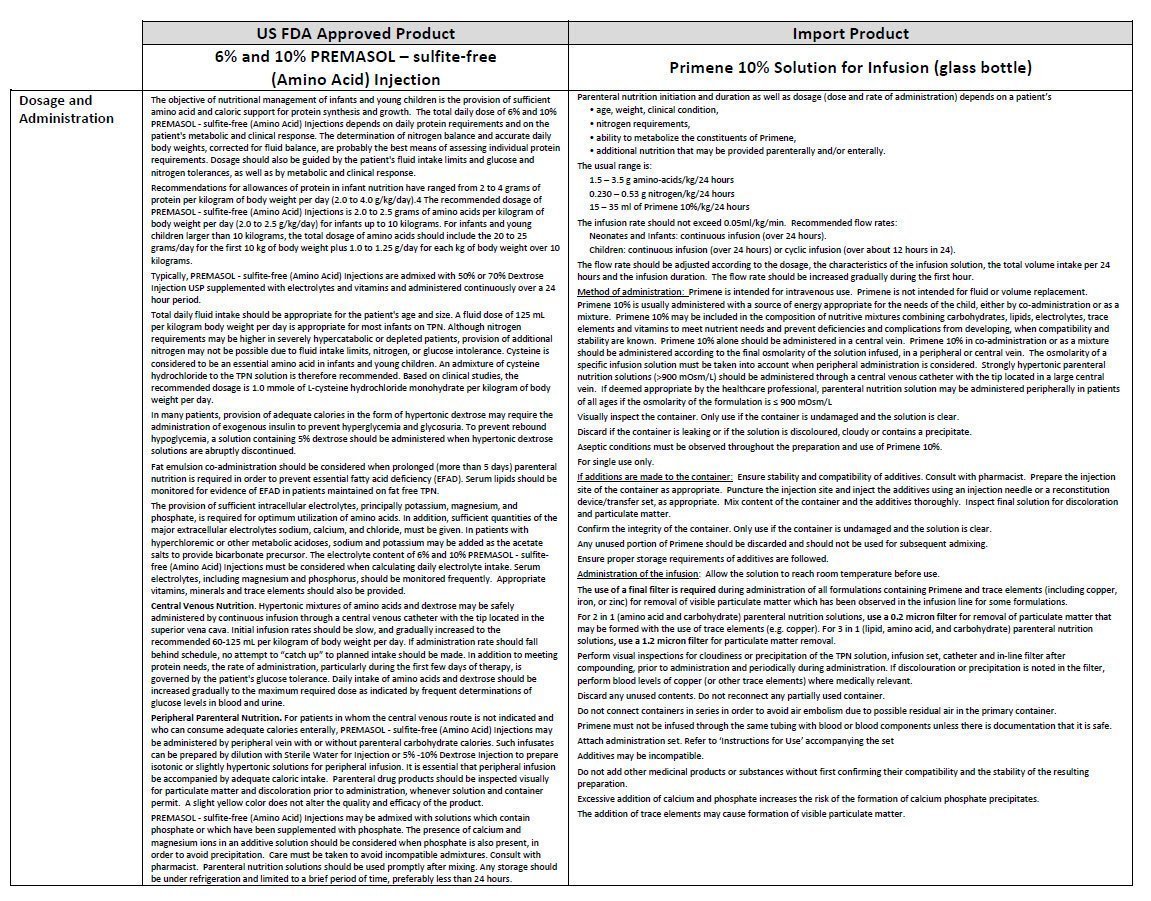
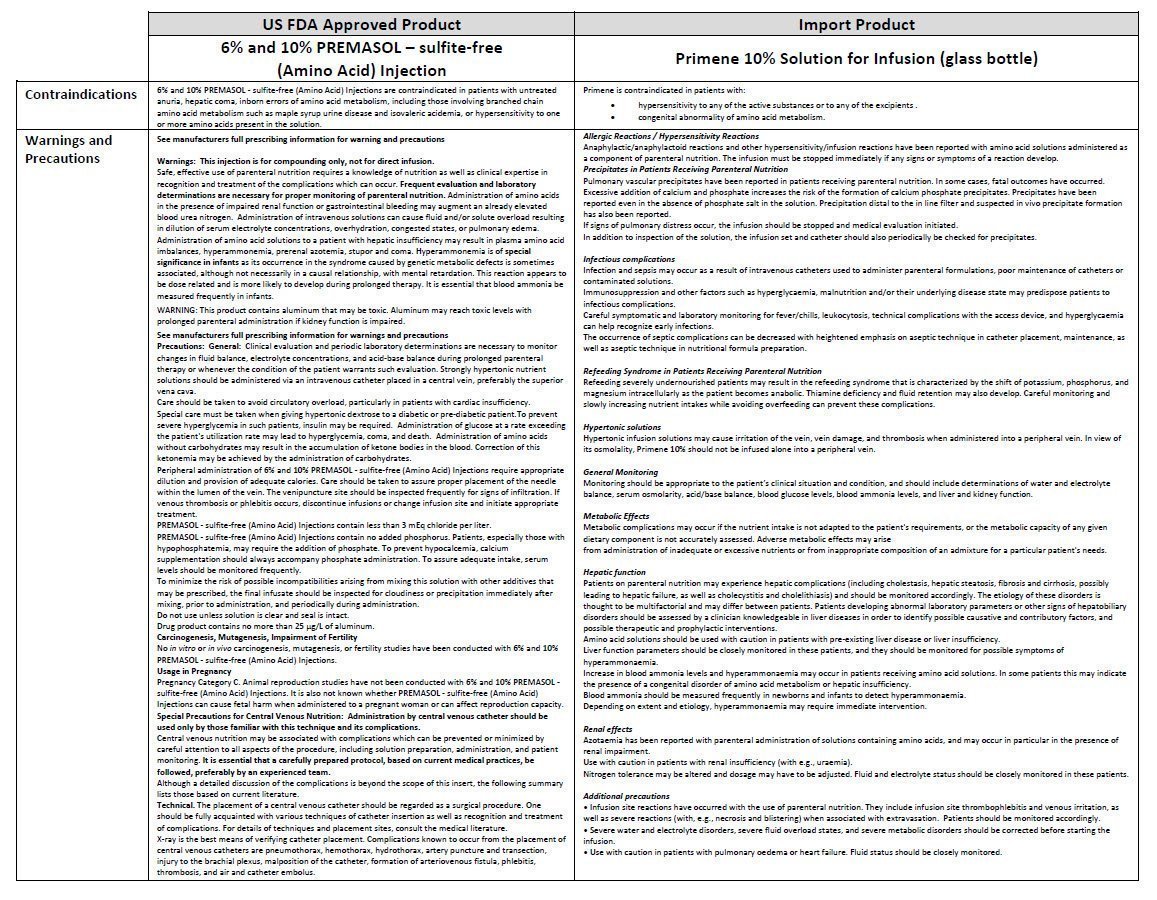
For intravenous use
- •
- Use as directed by physician.
- •
- WARNING: Hypertonic solution. Do not infuse alone into a peripheral vein.
- •
- Do not exceed the recommended infusion rate.
- •
- Do not store above 25°C. Protect from light.
- •
- Single use only. Discard any partly-used containers.
- •
- Do not use unless the vacuum is present.
- •
- Do not use if the bottle is leaking or the solution is discolured, cloudy or contains a precipitate.
- •
- Check compatibility and stability of nutritive mixtures before use.
- •
- Keep out of sight and reach of children.
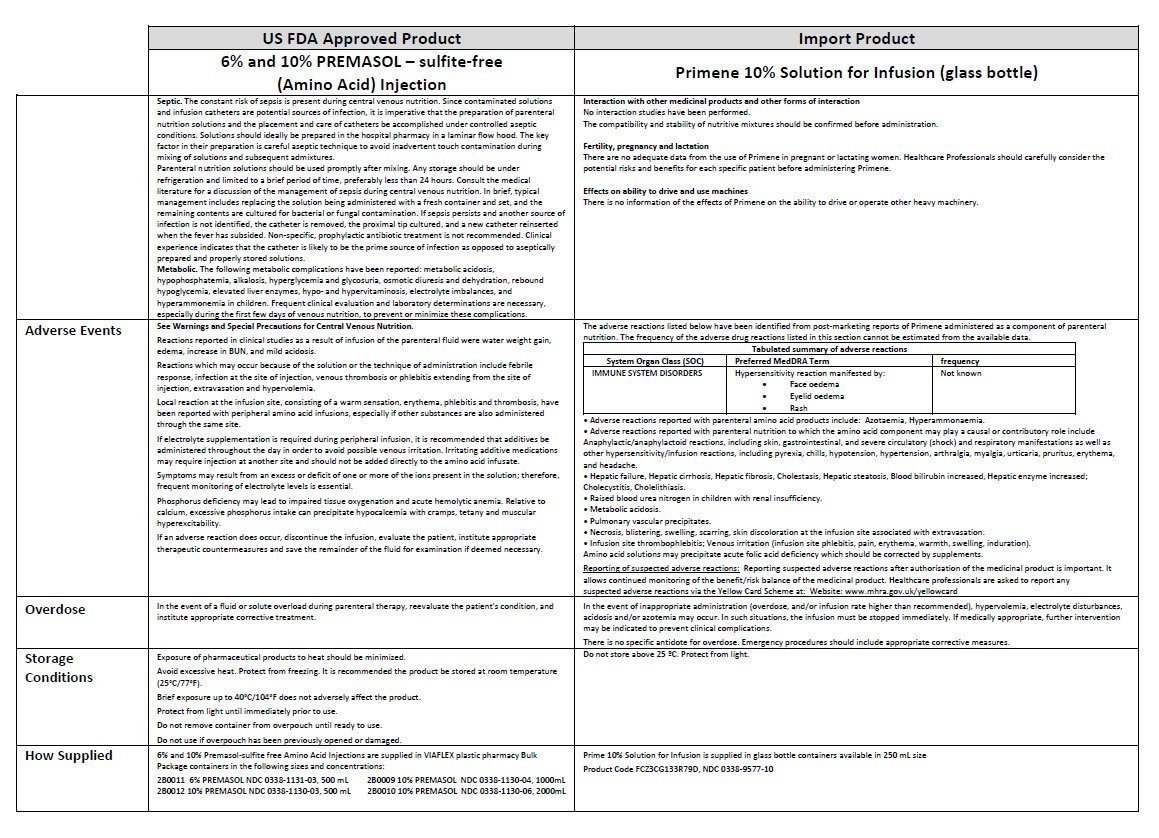
Baxter Healthcare Ltd., Caxton Way.
Thetford, Norfolk, IP24 3SE, UK
PL 0116/0283
POM symbol
Batch No:
Mfg. date:
Expiry date:
25/225
50/200
75/175
100/150
| PRIMENE
isoleucine, leucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, arginine, histidine, alanine, aspartic acid, cysteine, glutamic acid, glycine, proline, serine, tyrosine, ornitine hydrchloride, taurine injection, solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BIEFFE MEDITAL SPA | 514069459 | ANALYSIS(0338-9577) , MANUFACTURE(0338-9577) , LABEL(0338-9577) , PACK(0338-9577) , STERILIZE(0338-9577) | |
More about parenteral nutrition solution
- Check interactions
- Compare alternatives
- Pricing & coupons
- Latest FDA alerts (2)
- Side effects
- Drug class: intravenous nutritional products
Patient resources
Professional resources
Other brands
Hepatamine, Novamine, FreAmine HBC, Plenamine, ... +5 more