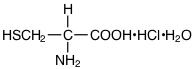L-Cysteine Hydrochloride Injection: Package Insert / Prescribing Info
Package insert / product label
Dosage form: injection, solution
Drug class: Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Jan 6, 2025.
On This Page
L-Cysteine Hydrochloride Injection Description
L-Cysteine Hydrochloride Injection, USP, 50 mg/mL, is a sterile, nonpyrogenic solution. Each mL contains: 50 mg of L-Cysteine Hydrochloride Monohydrate USP; Water for Injection, USP q.s.; Air replaced with Nitrogen. pH 1.0-2.5
L-Cysteine is a sulfur-containing amino acid. In premixed solutions of crystalline amino acids, cysteine is relatively unstable over time, eventually converting to insoluble cystine. To avoid such precipitation, L-Cysteine Hydrochloride Injection USP is intended to be used as an additive with Crystalline Amino Acid Injections immediately prior to administration to the patient.
The structural formula of Cysteine Hydrochloride Monohydrate USP is:
|
Molecular Weight |
Molecular Formula |
|
175.63 |
C3H7NO2S•HCl•H2O |
L-Cysteine Hydrochloride Injection - Clinical Pharmacology
L-Cysteine is synthesized from methionine via the trans-sulfuration pathway in the adult, but newborn infants lack the enzyme necessary to effect this conversion. Therefore, L-Cysteine is generally considered to be an essential amino acid in infants.
Indications and Usage for L-Cysteine Hydrochloride Injection
L-Cysteine Hydrochloride Injection, USP is intended for use only after dilution as an additive to Crystalline Amino Acid Injections to meet the intravenous amino acid nutritional requirements of infants receiving total parenteral nutrition.
Contraindications
This preparation should not be used in patients with hepatic coma or metabolic disorders involving impaired nitrogen utilization.
Warnings
Peripheral intravenous infusion of amino acids may induce a rise in blood urea nitrogen (BUN) especially in patients with impaired hepatic or renal function. Appropriate laboratory tests should be performed periodically and infusion discontinued if BUN levels exceed normal postprandial limits and continue to rise. It should be noted that a modest rise in BUN normally occurs as a result of increased protein intake.
Administration of amino acid solutions to a patient with hepatic insufficiency may result in serum amino acid imbalances, metabolic alkalosis, prerenal azotemia, hyperammonemia, stupor and coma.
Administration of amino acid solutions in the presence of impaired renal function may augment an increasing BUN, as does any protein dietary component.
Solutions containing sodium ion should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.
Solutions which contain potassium ion should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
Solutions containing acetate ion should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion such as severe hepatic insufficiency.
Hyperammonemia is of special significance in infants, as it can result in mental retardation. Therefore it is essential that blood ammonia levels be measured frequently in infants.
Instances of asymptomatic hyperammonemia have been reported in patients without overt liver dysfunction. The mechanisms of this reaction are not clearly defined but may involve genetic defects and immature or subclinically impaired liver function.
Frequent Clinical Evaluation and Laboratory Determinations are Necessary for Proper Monitoring During Administration. Blood studies should include glucose, urea nitrogen, serum electrolytes, ammonia, cholesterol, acid-base balance, serum proteins, kidney and liver function tests, osmolarity and hemogram. White blood count and blood cultures are to be determined if indicated. Urinary osmolarity and glucose should be determined frequently.
Safe use during pregnancy has not been established, therefore, infusion of amino acids should be undertaken during pregnancy only when this is deemed essential to the patients' welfare, as judged by the physician.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration
Precautions
Special care must be taken when administering hypertonic glucose to provide calories in diabetic or prediabetic patients.
Because of its antianabolic activity, concurrent administration of tetracycline may reduce the nitrogen sparing effects of infused amino acids.
Do not withdraw venous blood for blood chemistries through the peripheral infusion site, as interference with estimations of nitrogen containing substances may occur.
Intravenous feeding regimens which include amino acids should be used with caution in patients with a history of renal disease, pulmonary disease, or with cardiac insufficiency so as to avoid excessive fluid accumulation.
The effect of infusion of amino acids, without dextrose, upon carbohydrate metabolism of children is not known at this time.
Nitrogen intake should be carefully monitored in patients with impaired renal function. For long-term total nutrition, or if a patient has inadequate fat stores, it is essential to provide adequate exogenous calories concurrently with the amino acids. Concentrated dextrose solutions are an effective source of such calories. Such strongly hypertonic nutrient solutions should be administered through an indwelling intravenous catheter with the tip located in the superior vena cava.
Adverse Reactions/Side Effects
Local reactions consisting of a warm sensation, erythema, phlebitis and thrombosis at the infusion site have occurred with peripheral intravenous infusion of amino acids, particularly if the other substances, such as antibiotics, are also administered through the same site. In such cases the infusion site should be changed promptly to another vein. Use of large peripheral veins, inline filters, and slowing the rate of infusion may reduce the incidence of local venous irritation. Electrolyte additives should be spread throughout the day. Irritating additive medications may need to be injected at another venous site.
Generalized flushing, fever and nausea also have been reported during peripheral infusions of amino acid solutions.
L-Cysteine Hydrochloride Injection Dosage and Administration
L-Cysteine Hydrochloride Injection USP is intended for use only after dilution in Crystalline Amino Acid Injection. Each 0.5 gram of L-Cysteine Hydrochloride Monohydrate should be combined aseptically with 12.5 grams of Crystalline Amino Acid Injection, such as that present in 250 mL of 5% Crystalline Amino Acid Injection. The admixture is then diluted with 250 mL of dextrose 50% or such lesser volume as indicated. Equal volumes of 5% Crystalline Amino Acid Injection and dextrose 50% produce a final solution which contains Crystalline Amino Acid Injection 2.5% in dextrose 25%, which is suitable for administration by central venous infusion. Administration of the final admixture should begin within one hour of mixing. Otherwise, the admixture should be refrigerated immediately and used within 24 hours of the time of mixing. For the recommended rate of administration, see the Crystalline Amino Acid Injection package insert.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
How is L-Cysteine Hydrochloride Injection supplied
L-Cysteine Hydrochloride Injection, USP (50 mg/mL) is supplied as follows:
|
NDC Number |
Volume |
|
0781-8940-70 |
10 mL |
|
0781-8940-95 |
10 x 10 mL |
Store at controlled room temperature 15°-30°C (59°-86°F) Do not freeze.
For Sandoz Inc. Customer Service, call 1-800-525-8747.
Rx only
Manufactured in Canada by Avara Boucherville Pharmaceutical Services, Inc. for
Sandoz Inc., Princeton, NJ 08540
Product of Japan
Revised: August 2018
46236914
| L-CYSTEINE HYDROCHLORIDE
l-cysteine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sandoz Inc (005387188) |