1. Indications and Usage for Hemgenix
HEMGENIX is an adeno-associated virus vector-based gene therapy indicated for treatment of adults with Hemophilia B (congenital Factor IX deficiency) who:
- Currently use Factor IX prophylaxis therapy, or
- Have current or historical life-threatening hemorrhage, or
- Have repeated, serious spontaneous bleeding episodes.
2. Hemgenix Dosage and Administration
For single-use intravenous infusion only.
For patient selection:
- Perform Factor IX inhibitor titer testing.
In case of a positive test result for human Factor IX inhibitors, perform a re-test within approximately 2 weeks. If both the initial test and re-test results are positive, do not administer HEMGENIX to this patient.
- Perform liver health assessments, including:
- Enzyme testing [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin)],
- Hepatic ultrasound and elastography.
In case of radiological liver abnormalities and/or sustained liver enzyme elevations, consider a consultation with hepatologist to assess eligibility for HEMGENIX [see Warnings and Precautions (5.2)].
2.1 Dose
The recommended dose of HEMGENIX is 2 × 1013 genome copies (gc) per kilogram (kg) of body weight (or 2 mL/kg body weight) administered as an intravenous infusion after dilution with 0.9% sodium chloride solution (normal saline) [see Dosage and Administration (2.2)].
Calculate the dose as follows:
| HEMGENIX dose (in mL) = patient body weight (in kilogram) × 2 | |
The multiplication factor 2 represents the per kilogram dose (2 × 1013 gc/kg) divided by the amount of genome copies per mL of the HEMGENIX solution (1 × 1013 gc/mL).
Number of HEMGENIX vials needed = HEMGENIX dose (in mL) divided by 10 (round up to next whole number of vials).
The division factor 10 represents the extractable volume of HEMGENIX from each vial (10 mL).
The total volume of the patient's HEMGENIX dose to be diluted may be less than the total volume of vials needed.
Example calculation for 72 kg patient
| Patient Weight | HEMGENIX dose (mL) (body weight multiplied by 2) | Number of Vials needed [HEMGENIX dose (mL) divided by 10, then rounded up] |
| 72 kg | 144 mL | 15 |
HEMGENIX can be administered only once.
2.2 Preparation
The vials are for single-dose only.
General precautions
- Prepare HEMGENIX using sterile technique under aseptic conditions, proper engineering controls (e.g., biological safety cabinet or isolator) and according to institutional policies.
- Do not expose HEMGENIX to the light of an ultraviolet radiation disinfection lamp.
- Confirm that the patient's identity matches with the patient-specific identifier number on the outer carton.
- Verify the required dose of HEMGENIX based on the patient's body weight.
- Confirm that the carton contains sufficient number of vials to prepare the diluted HEMGENIX patient-specific infusion bag.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Required supplies and materials:
Normal saline infusion bag(s)* of 500 mL (1 to 2 bags based on patient's body weight)
Labels** for the infusion bag(s) of 500 mL
IV Infusion line/drip chamber* primed with 0.9% normal saline
Infusion bag connector(s)
20 mL or larger Luer-lock syringes*
20 G Needles* or vial adaptors*
70% isopropyl alcohol
Sharps disposal container
The following Table shows the supplies and materials compatible with HEMGENIX:
| Component* | Material of Construction |
| MABS = Methyl methacrylate acrylonitrile butadiene styrene; PE = Polyethylene; PP = Polypropylene; PVC = Polyvinyl chloride; TOTM = Trioctyltrimellitate, Acrylonitrile butadiene styrene (ABS) |
Normal saline infusion bag
(0.9% normal saline) | PE/PP copolymer (PVC-free)
(Stability after dilution was established using PE/PP copolymer, PVC-free infusion bags with 0.9% normal saline.) |
| 20 G Needle | Stainless Steel |
| Vial adapter | PP, Silicone; PP, stainless; MABS, acrylic silicone; ABS |
| Luer-lock syringe | PP, Silicone |
| IV Infusion line/drip chamber | PVC/TOTM, PP/styrene-ethylene-butylene-styrene |
**Information to be included on the infusion bag label:
- Product name: Diluted Hemgenix
- Patient identifier
- Expiration date/time (24 h from the vial removal from refrigerator)
- Storage condition: Room Temperature [15-25 °C (59-77 °F] protected from light.
- Contains genetically modified organisms
- Number of infusion bag: 1 of 2 bags / 2 of 2 bags
Preparation of 0.9% normal saline infusion bags
- Prior to dilution, spike the infusion bag(s) of 0.9% normal saline solution with applicable connector.
- Connect a luer-lock syringe at the mixing adapter site of the applicable connector.
- Withdraw the volume equal to the calculated HEMGENIX dose (in mL) from the 500 mL infusion bag(s) of 0.9% normal saline solution. The volume to be withdrawn and number of infusion bag(s) needed will vary based on the patient body weight:
| Patient body weight | Number of 500 mL 0.9% normal saline infusion bag(s) required | Volume of saline solution to withdraw |
| Less than 120 kg body weight | One | Equal to the total HEMGENIX dose (in mL) from one bag |
| Equal to or more than 120 kg body weight | Two | Equal to the total HEMGENIX dose (in mL). Remove half of the dose equivalent volume from each of the two bags. |
HEMGENIX injection to the 0.9% normal saline infusion bags
-
-
- Dilute HEMGENIX with 0.9% normal saline solution only prior to administration.
- 4.
- Prior to dilution, inspect each of the HEMGENIX single-dose vials.
- If particulates, cloudiness, or discoloration is visible, DO NOT use the vial(s).
- 5.
- Gently swirl the vials 3 times (about 10 seconds) to homogenize the HEMGENIX suspension.
- To avoid foaming, DO NOT shake the HEMGENIX vial(s).
- 6.
- Remove the plastic flip-off cap from the vials and disinfect the rubber stopper with a sterilizing agent (for example sterile 70% isopropyl alcohol).
- 7.
- Withdraw HEMGENIX from each vial using a 20 G needle/vial adapter and syringe.
- Use recommended 20 mL luer-lock or larger syringe that is suitable for volume measuring and a needle.
- DO NOT use filter needles during preparation of HEMGENIX.
- Use a new needle/vial adapter and syringe for each HEMGENIX vial.
- Dispose of the needle and syringe in an appropriate container.
- 8.
- Slowly add the required HEMGENIX dose from the syringe(s) directly to the 0.9% normal saline solution in the infusion bag(s) (from step #3) to bring the total volume in each infusion bag back to 500 mL.
- DO NOT add HEMGENIX into the airspace of the bag to avoid foaming throughout this process.
- 9.
- Repeat steps 7 and 8 with additional needles/vial adaptors and syringes to inject the total calculated HEMGENIX volume to the infusion bag(s) required for the patient dose.
- 10.
- Gently invert the infusion bag(s) at least 3 times (about 10 seconds) to mix the solution and ensure even distribution of the diluted product.
- To avoid foaming, DO NOT shake the diluted HEMGENIX infusion bag(s).
- 11.
- Label the infusion bag(s).
- 12.
- Connect the infusion bag(s) to an infusion tube pre-filled with sterile 0.9% normal saline solution to reduce the risk of spillage and/or aerosol formation.
- 13.
- Transport the diluted HEMGENIX infusion bag(s) in the transport container/bag protected from light to the administration site, avoiding any shaking or excessive agitation.
2.3 Administration
Required supplies and materials for administration:
Winged intravenous needle or catheter set*
Infusion pump
0.2 mcm in-line filter*
Antiseptic skin preps
70% isopropyl alcohol wipes
Gauze and tape, or transparent dressing
Sharps disposal container
Virucidal agent to treat spill/spill kit
The following Table shows the supplies and materials compatible for infusion of HEMGENIX:
| Component* | Material of Construction |
| DEHP = Di(2-ethylhexyl)phthalate; DEHT = Di(2-ethylhexyl)terephthalate; MABS = Methyl methacrylate acrylonitrile butadiene styrene; PES = Polyether sulfone; PVC = Polyvinyl chloride |
| Winged IV needle or catheter set | PVC/TOTM, MABS |
| 0.2 mcm in-line filter | PES |
| Catheter | PVC/DEHT, Stainless steel |
Administer HEMGENIX as a single-dose intravenous infusion through a peripheral venous catheter:
- Visually inspect diluted HEMGENIX prior to administration. The diluted HEMGENIX should be clear and colorless.
- Use an integrated (in-line) 0.2 mcm filter made out of PES.
- Subsequently, connect the pre-filled IV infusion line/drip chamber to the main intravenous line which has been primed with sterile 0.9% normal saline solution prior to use.
- Infuse diluted HEMGENIX at a constant infusion rate of 500 mL/hour (8 mL/min).
- DO NOT administer HEMGENIX as an intravenous push or bolus.
- DO NOT infuse the diluted HEMGENIX solution in the same intravenous line with any other products.
- DO NOT use a central line or port.
In the event of an infusion reaction during administration [see Warnings and Precautions (5.1)]:
- The rate of infusion may be reduced or stopped, to manage the infusion reaction.
If the infusion is stopped, restart at a slower rate when the infusion reaction is resolved.
- If the infusion rate needs to be reduced, or stopped and restarted, HEMGENIX should be infused within 24 hours after the dose preparation [see How supplied/Storage and handling (16.2)].
- After the entire content of the bag(s) is infused, flush the IV infusion line/drip chamber at the same infusion rate with 0.9% normal saline solution to ensure all HEMGENIX is delivered.
- Treat spills of HEMGENIX with a virucidal agent with proven activity against non-enveloped viruses.
- Dispose of unused product and disposable materials that may have come in contact with HEMGENIX in accordance with local biosafety guidelines applicable for handling and disposal of the pharmaceutical waste.
Monitoring Post-Administration
Conduct the following tests after HEMGENIX administration [see Warnings and Precautions (5.2, 5.3, 5.4)]:
- Perform regular liver enzyme testing to monitor for liver enzyme elevations which may indicate immune-mediated hepatotoxicity:
- Monitor ALT and AST (transaminase) levels by testing weekly for 3 months following administration of HEMGENIX. Continue to monitor transaminases in all patients who developed liver enzyme elevations until liver enzymes return to baseline.
- In the event of ALT increase to above normal limits or to twice the patient`s baseline in the first 3 months post-dose, consider implementing a course of corticosteroids. For patients with clinically relevant ALT increases who need corticosteroid treatment, administer the recommended starting dose of 60 mg/day of oral prednisolone or prednisone, with a subsequent taper in response to normalization of the ALT levels (see Table 1):
Table 1. Prednisolone Treatment Applied in Clinical Studies With HEMGENIX:
| Timeline | *Prednisolone Oral Dose (mg/day) |
|
|
| Week 1 | 60 |
| Week 2 | 40 |
| Week 3 | 30 |
| Week 4 | 30 |
| Maintenance dose until ALT level returns to baseline level | 20 |
| Taper dose after ALT baseline level has been reached | Reduce daily dose by 5 mg/week |
In the clinical studies, the mean duration of corticosteroid use for elevated transaminases was 81.4 days [Standard Deviation (SD) 28.6] and ranged from 51 to 130 days [see Warnings and Precautions (5.2)].
- Monitor Factor IX activity (e.g., weekly for 3 months).
- Monitor patients regularly for their Factor IX activity, in particular when exogenous Factor IX is administered. It may take several weeks before improved hemostatic control becomes apparent after HEMGENIX infusion; therefore, continued hemostatic support with exogenous human Factor IX may be needed during the first weeks after HEMGENIX infusion [see Clinical Pharmacology (12.3)].
- The use of different assays may impact the test results; therefore, use the same assay and reagents to monitor patients over time, if feasible [see Monitoring Laboratory Tests (5.5)].
- Use of exogenous Factor IX concentrates before and after HEMGENIX administration may impede assessment of endogenous, HEMGENIX-derived Factor IX activity.
- Perform regular alpha-fetoprotein (AFP) level testing and abdominal ultrasound (e.g., annually) in patients with preexisting risk factors for hepatocellular carcinoma (e.g., in patients with cirrhosis, advanced hepatic fibrosis, hepatitis B or C, non-alcoholic fatty liver disease (NAFLD), chronic alcohol consumption, non-alcoholic steatohepatitis (NASH), and advanced age).
- Monitor patients for human Factor IX inhibitors. Post-dose inhibitor testing should be performed if bleeding is not controlled, or plasma Factor IX activity levels decrease [see Warnings and Precautions (5.5)].
3. Dosage Forms and Strengths
HEMGENIX is a clear and colorless suspension for intravenous infusion.
HEMGENIX is provided in a kit containing 10 to 48 vials. Each kit constitutes a dosage unit based on the patient's body weight.
HEMGENIX has a nominal concentration of 1 × 1013 gc/mL, and each vial contains an extractable volume of not less than 10 mL.
4. Contraindications
None.
5. Warnings and Precautions
5.1 Infusion Reactions
Infusion reactions, including hypersensitivity reactions and anaphylaxis, may occur. Symptoms may include chest tightness, headaches, abdominal pain, lightheadedness, flu-like symptoms, shivering, flushing, rash, and hypertension. Closely monitor patients for signs or symptoms of an infusion reaction throughout the infusion period and for at least 3 hours after end of infusion. Do not infuse the product faster than 500 mL/hour [see Adverse Reactions (6)].
In the event of an infusion reaction during administration, the infusion may be slowed or stopped. If the infusion is stopped, restart at a slower rate when the infusion reaction has resolved. Consider treatment with a corticosteroid or antihistamine for management of an infusion reaction [see Dosage and Administration (2.1)].
5.2 Hepatotoxicity
Intravenous administration of a liver-directed AAV vector could potentially lead to liver transaminase elevations (transaminitis). Transaminitis, particularly when observed in the first 3 months after HEMGENIX administration, is presumed to occur due to immune-mediated injury of transduced hepatocytes and may reduce the therapeutic efficacy of the AAV-vector based gene therapy.
In clinical studies with HEMGENIX, most subjects had asymptomatic, and predominantly mild elevations in transaminases. Elevated ALT levels occurred most often in the first 4 months after HEMGENIX administration. There were some subjects who had a late onset of elevated ALT levels between Months 6-24 (range = 42 IU/L-193 IU/L); however, all of these ALT values were <2× ULN with the exception of one subject. Three additional subjects had AST elevations with onset and resolution between Months 6 and 12 (range = 41 IU/L – 96 IU/L).
In one subject, an ALT elevation >5× ULN occurred 24 days after HEMGENIX administration and resolved by 51 days post-HEMGENIX administration. There was one subject who had an AST elevation > 5× ULN that occurred 11 months post-HEMGENIX administration and resolved to <2× ULN eight days later.
The majority of the elevated ALT values returned to baseline, however 9 subjects' ALT values never resolved to normal (range at 2-year follow up = 48 IU/L – 193 IU/L) [see Adverse Reactions (6)].
Closely monitor transaminase levels once per week for 3 months after HEMGENIX administration to mitigate the risk of potential hepatotoxicity. Continue to monitor transaminases in all patients who developed liver enzyme elevations until liver enzymes return to baseline [see Dosage and Administration (2.3)].
In case of increased ALT levels above the upper limit of normal or double baseline levels, consider implementing a course of corticosteroid, along with human Factor IX activity monitoring [see Dosage and Administration (2.3)].
5.3 Immune-mediated neutralization of the AAV5 vector capsid
In AAV-vector based gene therapies, preexisting neutralizing anti-AAV antibodies may impede transgene expression at desired therapeutic levels. Following treatment with HEMGENIX all subjects developed neutralizing anti-AAV antibodies. Currently, there is no validated neutralizing anti-AAV5 antibody assay.
In the clinical studies with HEMGENIX, an unvalidated clinical trial assay was utilized to assess preexisting neutralizing anti-AAV5 antibodies. The subject sub-group with detectable preexisting neutralizing anti-AAV5 antibodies up to titers of 1:678 showed mean Factor IX activity that was numerically lower compared to that subject sub-group without detectable preexisting neutralizing anti-AAV5 antibodies. Subjects, with and without preexisting neutralizing anti-AAV5 antibodies, demonstrated hemostatic protection. In one subject with a preexisting neutralizing anti-AAV5 antibody titer of 1:3212, no human Factor IX expression was observed, and restart of the exogenous Factor IX prophylaxis was needed for bleeding events. [see Clinical Studies (14)].
Anti-AAV5 Antibody Study
Patients who intend to receive treatment with HEMGENIX are encouraged to enroll in a study to measure pre-existing anti-AAV5 neutralizing antibodies by calling CSL Behring at 1-800-504-5434. The study evaluates the effect of pre-existing anti-AAV5 neutralizing antibodies on the risk of bleeding.
5.4 Hepatocellular carcinogenicity
The integration of liver-targeting AAV vector DNA into the genome may carry the theoretical risk of hepatocellular carcinoma development.
HEMGENIX is composed of a non-replicating AAV5 vector whose DNA persists largely in episomal form. Random integration of HEMGENIX vector DNA to the human DNA at low frequency is possible. No HEMGENIX-associated clonal expansion or carcinogenicity was observed in clinical studies [see Clinical Studies (14)]. One subject with preexisting risk factors for developing hepatic cancer developed a hepatocellular carcinoma, which was assessed as not likely related to HEMGENIX treatment based on vector integration site analyses and whole genome sequencing.
Patients with preexisting risk factors for hepatocellular carcinoma (e.g., patients with cirrhosis, advanced hepatic fibrosis, hepatitis C or B, non-alcoholic fatty liver disease (NAFLD), chronic alcohol consumption, non-alcoholic steatohepatitis (NASH), and advanced age) should receive abdominal ultrasound screenings and be monitored regularly (e.g., annually) for alpha-fetoprotein (AFP) elevations in the 5 years following administration [see Dosage and Administration (2.3)].
5.5 Monitoring Laboratory Tests
After HEMGENIX administration, regularly monitor patient's Factor IX activity levels.
When using an in vitro activated partial thromboplastin time (aPTT)-based one-stage clotting assay (OSA) for determining Factor IX activity, plasma Factor IX activity results can be affected by both the type of aPTT reagent and the reference standard used in the assay. This is important to consider particularly when changing the laboratory and/or reagents used in the assay. Therefore, the same assay and reagents are recommended to be used to monitor Factor IX activity over time.
The results of Factor IX activity tests are lower if measured with chromogenic substrate assay (CSA) compared to OSA.
In the clinical efficacy study with HEMGENIX, the post-dose Factor IX activity measured with CSA returned lower values with the mean CSA to OSA Factor IX activity ratio ranging from 0.41 to 0.55.
Monitor patients through appropriate clinical observations and laboratory tests for the development of inhibitors to Factor IX after HEMGENIX administration. Perform an assay that detects Factor IX inhibitors if bleeding is not controlled, or plasma Factor IX activity levels decrease.
6. Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥5%) reported in clinical studies were ALT elevations, headache, blood creatine kinase elevations, flu-like symptoms, infusion-related reactions, fatigue, malaise, and AST elevations.
The following adverse reactions are discussed in greater detail in other sections of the label:
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of HEMGENIX was evaluated in two clinical studies (the first study enrolled 3 subjects and the second study 54 subjects). Both studies enrolled adult male subjects with moderately severe or severe Hemophilia B (N = 57), who received a single intravenous dose of 2 × 1013 gc/kg body weight of HEMGENIX. All subjects entered a follow-up period of 5 years.
No serious adverse reactions were reported [see Clinical Studies (14)]. The most common adverse reactions observed in ≥5% of subjects post-dose are listed in Table 2:
Table 2. Adverse Reactions (Incidence ≥5%) Following Treatment with HEMGENIX
| Adverse Reactions ≥5% | Subjects (%) (N = 57) |
|
|
| Alanine aminotransferase increased | 24 (42%) |
| Headache | 10 (18%) |
| Blood creatine kinase increased | 24 (42%) |
| Flu-like symptoms | 8 (14%) |
| Infusion-related reactions* (see below) | 19* (33%) |
| Hypersensitivity | 2† (4%) |
| Fatigue | 7 (12%) |
| Aspartate aminotransferase increased | 24 (42%) |
| Nausea | 4 (7%) |
| Malaise | 7 (12%) |
Infusion-related reactions were observed in 19 subjects. Infusions were temporarily interrupted in 3 subjects and resumed at a slower infusion rate after treatment with antihistamines and/or corticosteroids. In one subject, infusion was stopped and not resumed (see footnote of Table 2).
There were 24 subjects who had elevated ALT values from Day 8 to 731 post-administration.
Five subjects had ALT elevations >2-3× ULN (range = 89 IU/L – 130 IU/L), one subject had an ALT elevation > 3-5× ULN (193 IU/L) and one subject had an ALT elevation > 5× ULN (275 IU/L). The subject who had the ALT elevation >5× ULN occurred 3 weeks after HEMGENIX administration.
Five subjects had AST elevations > 2-3× ULN (range = 71 IU/L – 118 IU/L), three subjects had AST elevations > 3-5× ULN (range = 127 IU/L – 163 IU/L) and one subject had an AST elevation > 5× ULN (327 IU/L). The subject who had the AST elevation > 5× ULN occurred 11 months post-HEMGENIX administration.
Seventeen subjects had elevations in ALT levels within the first 4 months after HEMGENIX infusion (range = 41 IU/L – 275 IU/L), eleven of these subjects' ALT levels resolved within 4 months post-infusion (range = 41 IU/L – 275 IU/L) and five of these subjects' ALT levels never normalized as of last follow-up (range of values at 2-year follow-up = 48 IU/L – 110 IU/L). Seven additional subjects had ALT elevations with onset between Months 6 to 24 (range = 42 IU/L-193 IU/L), five of these subjects had additional risk factors for having elevated transaminase levels including Hepatitis C and Human Immunodeficiency Virus (HIV). ALT levels never normalized as of last follow-up (range of values at 2-year follow-up = (59 IU/L- 193 IU/L) in three of the subjects with ALT elevations with onset between Months 6 to 24.
Nineteen subjects had elevations in AST levels within 3 months after HEMGENIX infusion (range = 32 IU/L- 163 IU/L). Nine of these subjects' AST elevations resolved within 4 months post-infusion (range = 35 IU/L – 163 IU/L), three resolved within 7 to 13 months post-infusion (range = 35 IU/L – 62 IU/L), and seven of these subjects' AST levels never normalized as of last follow-up (range of values at 2-year follow-up = 36 IU/L – 327 IU/L). The remaining 5 subjects with AST elevation had onset of between 6 months and 2 years post-infusion (range = 36 IU/L – 127 IU/L), and AST levels had not normalized as of the last follow-up for one subject (AST at 2-year follow-up = 127 IU/L) who had additional risk factors for having elevated transaminase levels.
Nine subjects with ALT elevations received a tapered course of corticosteroids. The mean duration of corticosteroid treatment for the elevated ALT was 81.4 days. Nineteen of the 24 subjects with ALT elevations also had a related AST elevation. Twenty-one subjects had elevated transaminase levels and were not treated with corticosteroids. [see Clinical Studies (14)].
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
HEMGENIX is not intended for administration in women. No adverse effects on mating rate and fertility indices or fetal weights were observed in healthy naïve female mice mated with healthy male mice that were intravenously administered a predecessor of HEMGENIX product 6 days prior to mating. Vector DNA was not detected in the uterus, placenta, or fetus.
In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
HEMGENIX is not intended for administration in women.
8.3 Females and Males of Reproductive Potential
Risk Summary
No clinical studies have been performed to evaluate the effects of HEMGENIX on fertility in humans. Twenty days after intravenous administration of a predecessor of HEMGENIX product in healthy male mice, vector DNA was detected in all reproductive tissues examined (epididymis, seminal vesicles, testes, and sperm). However, no differences were observed in mating rates and fertility indices in healthy naïve female mice following mating with the dosed males.
8.4 Pediatric Use
The safety and efficacy of HEMGENIX in pediatric patients have not been established.
8.5 Geriatric Use
The clinical studies included a total of 6 geriatric subjects with Hemophilia B, aged 68 to 75 years at time of enrollment. No meaningful differences in the safety and efficacy profile were observed in these subjects compared to subjects aged 18 to 65 years, and no dose adjustment was made [see Clinical Studies (14)].
8.6 Hepatic Impairment
Limited clinical data in subjects with liver impairment indicate numerically lower FIX activity as compared to subjects without hepatic impairment [see Clinical Pharmacology (12.3)]. In the clinical studies, no dose adjustment was made in subjects with hepatic pathologies. The safety and efficacy in subjects with advanced hepatic impairment, including cirrhosis, advanced liver fibrosis, or uncontrolled Hepatitis B and C, have not been studied.
8.7 Renal Impairment
Limited clinical data are available in subjects with mild and moderate renal impairment [see Clinical Pharmacology (12.3)]. In the clinical studies, no dose adjustment was made in these subjects. The safety and efficacy in subjects with severe renal impairment and end-stage renal disease have not been studied.
11. Hemgenix Description
HEMGENIX (etranacogene dezaparvovec-drlb) is an adeno-associated viral vector-based gene therapy for intravenous infusion after dilution. HEMGENIX is a non-replicating recombinant AAV5 containing a codon-optimized DNA sequence of the gain-of-function Padua variant of human Factor IX (variant R338L), under control of a liver-specific promotor 1 (LP1).
HEMGENIX has a nominal concentration of 1 × 1013 gc/mL. Each vial contains an extractable volume of no less than 10 mL of HEMGENIX and the following excipients: sucrose (50 mg/mL), polysorbate-20 (0.22 mg/mL), potassium chloride (0.2 mg/mL), potassium phosphate (0.2 mg/mL), sodium chloride (8 mg/mL), and sodium phosphate (1.2 mg/mL).
HEMGENIX is sterile, clear and colorless suspension, and contains no preservative. After dilution, HEMGENIX should be clear and colorless suspension.
12. Hemgenix - Clinical Pharmacology
12.1 Mechanism of Action
HEMGENIX is an adeno-associated virus serotype 5 (AAV5) based gene therapy designed to deliver a copy of a gene encoding the Padua variant of human coagulation Factor IX (hFIX-Padua). Single intravenous infusion of HEMGENIX results in cell transduction and increase in circulating Factor IX activity in patients with Hemophilia B.
12.2 Pharmacodynamics
Factor IX activity
The mean Factor IX activity levels over time, as measured by one-stage [activated Partial Thromboplastin Time (aPTT)-based] assay are summarized in Table 3. Subjects achieved a mean (± SD) uncontaminated (i.e., excluding measurements within five half-lives of Factor IX replacement therapy) Factor IX activity levels of 39% (± 18.7), 41.5% (± 21.7), 36.9% (± 21.4) and 36.7 (± 19.0) of normal, respectively, at 6, 12, 18 and 24 months. The time to onset of Factor IX protein expression post-dose was detectable by first uncontaminated measurement at Week 3 in the clinical efficacy study (N = 54) [see Clinical Studies (14)].
Table 3: Summary of Uncontaminated Factor IX Activity Over Time Following Administration of 2 × 1013 gc/kg of HEMGENIX [FAS; One-Stage (aPTT-Based) Assay]
| Factor IX Activity in % (One-stage) |
| Subject Number (*n) | Median (Min, Max) | Mean (SD) |
| Abbreviations: SD = Standard Deviation; FAS = Full Analysis Set including all 54 subjects dosed; Min = Minimum; Max = Maximum. Uncontaminated Factor IX activity values exclude measurements within five half-lives of Factor IX replacement therapy. |
|
|
| Week 3 | 43 | 23.7 (4.9, 56.7) | 26.8 (12.7) |
| Month 3 | 51 | 33.8 (7.6, 91.0) | 36.8 (18.2) |
| Month 6 | 51 | 37.3 (8.2, 97.1) | 39.0 (18.7) |
| Month 12 | 50 | 39.9 (5.9, 113.0) | 41.5 (21.7) |
| Month 18 | 50 | 33.6 (4.5, 122.9) | 36.9 (21.4) |
| Month 24 | 50 | 33.9 (4.7, 99.2) | 36.7 (19.0) |
Pharmacodynamics in specific populations
Age
Limited data (N = 7) from 60 -75 years subgroup showed that the mean Factor IX activity levels were approximately up to 2-fold higher in this subgroup compared to 18 to < 40 years age subgroup (N = 31), but comparable to 40 to <60 years age subgroup (N = 15).
Hepatic Impairment
In the clinical efficacy study, subjects with varying degree of baseline liver pathology, specifically the degree of hepatic steatosis with the Controlled Attenuation Parameter (CAP) score of ≥S2 (≥260 decibels/m; range: 262 to 400; n = 12) versus <S2 (<260 decibels/m; range: 100 to 259; n = 28;) and missing score (n = 14) were compared [see Clinical Studies (14)]. The mean (± SD) uncontaminated Factor IX activity for <S2 versus ≥S2 subgroups at Months 6, 12, 18, and 24 post dose were 40.8 (±20.1) versus 34.5 (±13.7), 46.4 (±24.1) versus 32.6 (±18.6), 41.6 (±25.7) versus 29.2 (±13.7), and 40.2 (±19.8) versus 28.4 (±13.1), respectively.
Subjects with advanced liver impairment and advanced fibrosis (elastography of e.g., ≥9 kPA, or suggestive of or equal to METAVIR Stage 3 disease), were not studied.
Renal Impairment
In the clinical efficacy study, subjects with mild renal impairment (creatinine clearance (CLcr) = 60 to 89 mL/min defined by Cockcroft-Gault equation, n = 7) had about 37% higher Factor IX activity relative to those with normal renal function (CLcr ≥90 mL/min; n = 45) following HEMGENIX administration. One subject with moderate renal impairment (CLcr = 30 to 59 mL/min) had similar Factor IX activity as subjects with normal renal function.
HEMGENIX was not studied in subjects with severe renal impairment (CLcr = 15 to 29 mL/min) or end-stage renal disease (CLcr< 15 mL/min).
12.3 Pharmacokinetics
Vector Biodistribution (within the body) and Vector Shedding (excretion/secretion)
Nonclinical data
Biodistribution of HEMGENIX was evaluated after intravenous administration in healthy male mice and non-human primates (NHPs). The highest levels of vector DNA were detected in the liver and adrenal glands in both species. Vector DNA was also detected in all reproductive tissues examined (epididymis, seminal vesicles, and testes). In a mating study evaluating a predecessor of HEMGENIX, transmission of vector DNA to naïve female mice following mating with dosed males was not observed [see Nonclinical Toxicology (13.2)].
Clinical data
Following administration of the predecessor of HEMGENIX at doses of 5 ×1012 (N = 5) and 2 × 1013 gc/kg (N = 5) in a clinical study, the pharmacokinetics of vector DNA in blood and viral shedding in saliva, nasal secretions, semen, urine, and feces were characterized. Clearance of vector DNA as confirmed by 3 subsequent measurements below limit of detection (LOD), was achieved in all subjects at both dose levels from all the matrices except for semen, where clearance was achieved in 9/10 subjects. One subject was unable to produce semen due to a historical medical condition and, therefore, shedding from semen could not be assessed. The maximum time to clearance of vector DNA was 22 weeks for urine, 26 weeks for saliva and nasal secretions, 40 weeks for feces, 52 weeks for semen, and 159 weeks for blood.
Subsequently, the pharmacokinetics of vector DNA in blood, and viral shedding in semen following HEMGENIX administration was characterized in 2 clinical studies.
In an initial clinical study (N = 3), clearance of vector DNA from semen and blood (i.e., confirmed with 3 subsequent measurements below LOD of vector DNA) was achieved in 2/3 subjects, and in all subjects, respectively, after 3 years post-administration. One subject did not return the required number of semen samples to assess the shedding status as per the definition of 3 subsequent measurements below LOD of vector DNA.
In the clinical efficacy study (N = 54), a total of 56% (30/54) of subjects achieved absence of vector DNA from blood and 69% (37/54) from semen by Month 24. Several subjects did not return the required number of blood and semen samples to assess the shedding status as per the definition of 3 subsequent measurements below LOD of vector DNA. Considering results obtained from 2 available consecutive samples below LOD, a total of 40/54 (74%) and 47/54 (87%) subjects were identified to have reached absence of vector DNA from blood and semen, respectively, at 24 months post-administration.
12.6 Immunogenicity
In clinical studies sustained humoral immune response to infused AAV5 capsid was observed in all subjects following treatment with HEMGENIX. The neutralizing anti-AAV5 antibody levels raised above the upper limit of quantification by week 3 post-administration and remained elevated, as measured at month 24 post-dose. Re-administration of HEMGENIX in the presence of high anti-AAV5 antibody titer has not been evaluated. Currently, there is no validated neutralizing anti-AAV5 antibody assay.
13. Nonclinical Toxicology
Nonclinical studies were initiated with a predecessor of HEMGENIX product, rAAV5 expressing the wild type human coagulation factor IX (rAAV5-hFIX). HEMGENIX was developed by introducing a 2-nucleotide change in the transgene for hFIX, generating the naturally occurring Padua variant of Factor IX (rAAV5-hFIX-Padua).
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No traditional nonclinical carcinogenicity or mutagenicity studies were conducted with HEMGENIX; such studies were not indicated. No adverse effects were observed in mating rates and fertility indices in healthy naïve female mice following mating with males that were administered the predecessor of HEMGENIX [see Use in specific populations (8.3)]. To evaluate vector integration, host genomic DNA was isolated from liver tissue obtained from healthy mice and NHPs following intravenous administration of the predecessor of HEMGENIX. For both species, the identified rAAV5-hFIX vector DNA sequences represented episomal forms that were not integrated into the host DNA. A low level of integrated rAAV5-hFIX DNA was distributed throughout the host genome with no predilection to specific integration sites, including in genes associated with malignant transformation in humans.
13.2 Animal Toxicology and/or Pharmacology
A pharmacology study was conducted in a murine model of Hemophilia B (B6.129P2-F9tm1Dws. Intravenous administration of the predecessor of HEMGENIX at dose levels ranging from 5×1011 to 2.3×1014 gc/kg, resulted in dose-dependent increases in plasma hFIX protein levels, plasma hFIX clotting activity, and vector transduction in the liver at 4 weeks post-dose.
Intravenous administration of HEMGENIX resulted in a no-observed-adverse-effect-level of 5 × 1013 gc/kg (the maximum dose level administered) in healthy mice and 9 × 1013 gc/kg in NHPs. Vector biodistribution to the liver and hFIX protein levels in the plasma occurred in a dose-dependent manner in both species. Anti-hFIX antibodies developed in 5 out of 12 NHPs administered HEMGENIX, which correlated with a decline in circulating hFIX protein levels beginning at 13 weeks post-dose.
One out of 10 healthy mice administered 5 × 1013 gc/kg of HEMGENIX or the predecessor of HEMGENIX developed pulmonary thrombi at 13 weeks post-dose. This dose level is 2.5-fold higher than the recommended dose level for HEMGENIX. Compared to concurrent controls, prolonged prothrombin time, decreased activated partial thromboplastin time and decreased heart rates were observed in NHPs administered 9 × 1013 gc/kg of HEMGENIX during the 26-week study. This dose level is 4.5-fold higher than the recommended dose level for HEMGENIX.
14. Clinical Studies
The efficacy of HEMGENIX was evaluated in a prospective, open-label, single-dose, single-arm, multi-national study (N = 54). The study enrolled adult male subjects aged 19 to 75 years, with severe or moderately severe Hemophilia B, who received a single intravenous dose of 2 × 1013 gc/kg body weight of HEMGENIX and entered a follow-up period of 5 years. The study is on-going.
The 54 subjects prospectively completed a lead-in period of at least six months with the intent to receive standard of care routine Factor IX prophylaxis. These 54 subjects then received the indicated single intravenous dose of HEMGENIX. Subjects were then followed up monthly until Month 12, and then at 6-month intervals until Year 5. For the efficacy evaluation, data up to 18 months post-treatment were used. Of the 54 subjects, 53 subjects completed at least 18 months of follow-up in the ongoing study. One subject with numerous cardiovascular and urologic risk factors, aged 75 years at screening, died of urosepsis and cardiogenic shock at Month 15 post-dose (at age 77 years) unrelated to treatment. Another subject received around 10% of the intended dose of HEMGENIX due to an infusion-related hypersensitivity reaction.
The main efficacy outcome was a non-inferiority test of annualized bleeding rate (ABR) during Months 7 to 18 after HEMGENIX treatment compared with ABR during the lead-in period. All bleeding episodes, regardless of investigator assessment, were counted. Subjects were allowed to continue prophylaxis during Months 0 to 6. The estimated mean ABR during Months 7 to 18 after HEMGENIX treatment was 1.9 bleeds/year with a 95% confidence interval (CI) of (1.0, 3.4), compared with an estimated mean ABR of 4.1 [95% CI: 3.2, 5.4] during the lead-in period. The ABR ratio (Months 7 to 18 post-treatment / lead-in) was 0.46 [95% CI: 0.26, 0.81], demonstrating non-inferiority of ABR during Months 7 to 18 compared to the lead-in period.
Two subjects were not able to stop routine prophylaxis after HEMGENIX treatment. During Months 7 to 18, an additional subject received prophylaxis from Days 396-534 [approximately 20 weeks].
Table 4. Total Bleeding Events and ABRs (Full Analysis Set: N=54)
| Lead-in Period* | Months 7 to 18† after HEMGENIX treatment |
| Abbreviations: ABR = Annualized Bleeding Rate; CI = Confidence Interval |
|
|
| All Bleeds | 136 | 96‡ |
| Follow-up time (Person-Year) | 33 | 52 |
| Mean Adjusted ABR (95% CI)§ | 4.1
(3.2, 5.4) | 1.9
(1.0, 3.4) |
| Subjects with Bleeds | 40 (74%) | 20 (37%) |
| Subjects with Zero Bleeds | 14 (26%) | 34 (63%) |
| Observed Spontaneous Bleed Count (Proportion of total bleeds)¶ | 50 (37%) | 14 (26%) |
| Observed Joint Bleed Count (Proportion of total bleeds)¶ | 77 (57%) | 19 (35%) |
After a single-dose of HEMGENIX, increases in Factor IX activity were observed [see Pharmacokinetics (12.3)].
16. How is Hemgenix supplied
16.1 How Supplied
HEMGENIX is supplied as sterile, preservative-free, clear, and colorless suspension. HEMGENIX has a nominal concentration of 1 × 1013 gc/mL.
HEMGENIX is provided as a customized kit to meet dosing requirements for each patient [see Dosage and Administration (2.1)], with each kit containing 10 (ten) to 48 (forty-eight) single-use vials (NDC 0053-0099-01), each with an extractable volume of no less than 10 mL of HEMGENIX (see5). The total number of vials in each kit corresponds to the dosing requirement for the individual patient depending on the patient`s body weight [see Dosage and Administration (2.1)]. The customized kit is accompanied with patient`s specific identifier number (Lot) on the outer carton. Each HEMGENIX kit may contain different drug product lots.
Kit sizes and National Drug Codes (NDC) are provided in Table 5:
Table 5. HEMGENIX Multi-Vial Kits
| Total Number of Vials per Kit | Patient Body Weight
(kg) | Total Volume per Kit
(mL) | NDC Number |
| 10 | 46-50 | 100 | 0053-0100-10 |
| 11 | 51-55 | 110 | 0053-0110-11 |
| 12 | 56-60 | 120 | 0053-0120-12 |
| 13 | 61-65 | 130 | 0053-0130-13 |
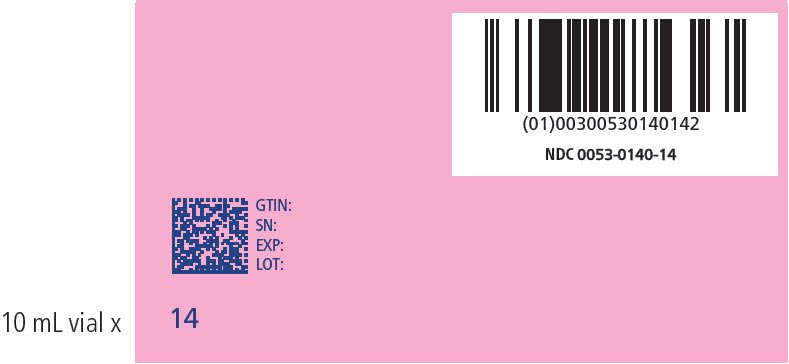
| 14 | 66-70 | 140 | 0053-0140-14 |
| 15 | 71-75 | 150 | 0053-0150-15 |
| 16 | 76-80 | 160 | 0053-0160-16 |
| 17 | 81-85 | 170 | 0053-0170-17 |
| 18 | 86-90 | 180 | 0053-0180-18 |
| 19 | 91-95 | 190 | 0053-0190-19 |
| 20 | 96-100 | 200 | 0053-0200-20 |
| 21 | 101-105 | 210 | 0053-0210-21 |
| 22 | 106-110 | 220 | 0053-0220-22 |
| 23 | 111-115 | 230 | 0053-0230-23 |
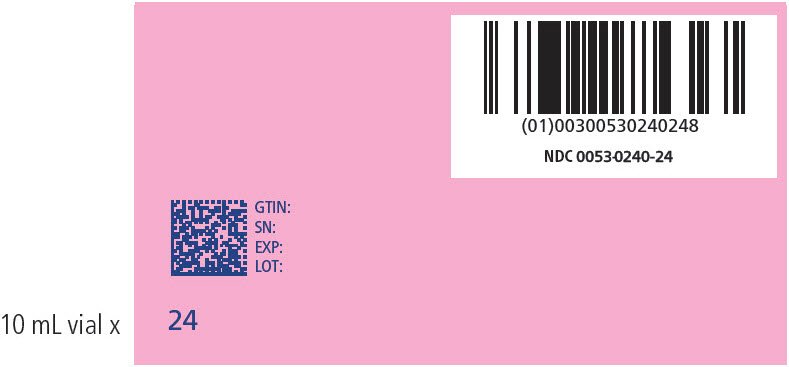
| 24 | 116-120 | 240 | 0053-0240-24 |
| 25 | 121-125 | 250 | 0053-0250-25 |
| 26 | 126-130 | 260 | 0053-0260-26 |
| 27 | 131-135 | 270 | 0053-0270-27 |
| 28 | 136-140 | 280 | 0053-0280-28 |
| 29 | 141-145 | 290 | 0053-0290-29 |
| 30 | 146-150 | 300 | 0053-0300-30 |
| 31 | 151-155 | 310 | 0053-0310-31 |
| 32 | 156-160 | 320 | 0053-0320-32 |
| 33 | 161-165 | 330 | 0053-0330-33 |
| 34 | 166-170 | 340 | 0053-0340-34 |
| 35 | 171-175 | 350 | 0053-0350-35 |
| 36 | 176-180 | 360 | 0053-0360-36 |
| 37 | 181-185 | 370 | 0053-0370-37 |
| 38 | 186-190 | 380 | 0053-0380-38 |
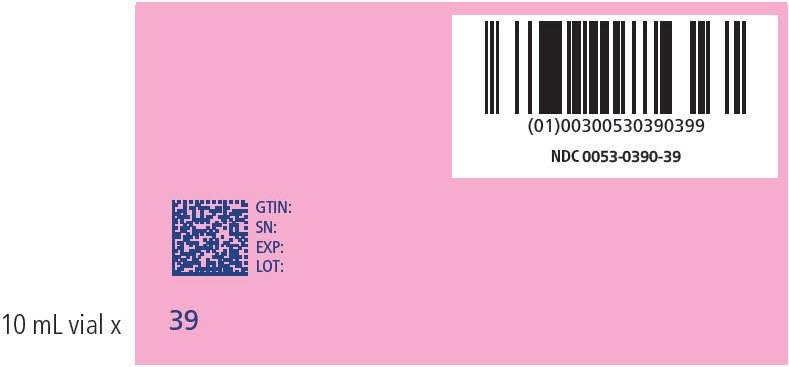
| 39 | 191-195 | 390 | 0053-0390-39 |
| 40 | 196-200 | 400 | 0053-0400-40 |
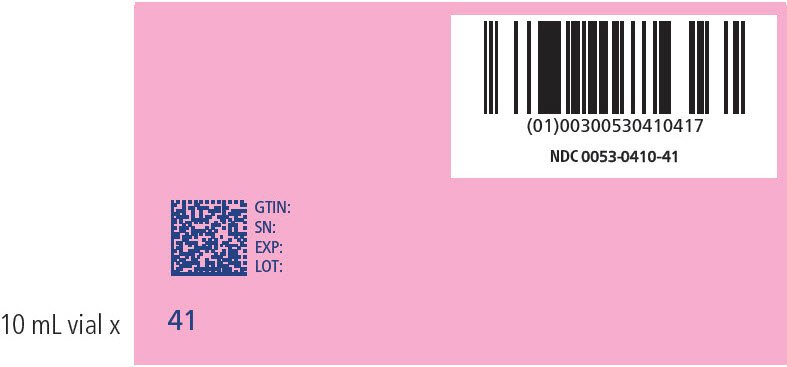
| 41 | 201-205 | 410 | 0053-0410-41 |
| 42 | 206-210 | 420 | 0053-0420-42 |
| 43 | 211-215 | 430 | 0053-0430-43 |
| 44 | 216-220 | 440 | 0053-0440-44 |
| 45 | 221-225 | 450 | 0053-0450-45 |
| 46 | 226-230 | 460 | 0053-0460-46 |
| 47 | 231-235 | 470 | 0053-0470-47 |
| 48 | 236-240 | 480 | 0053-0480-48 |
16.2 Storage and Handling
- HEMGENIX is shipped at 2°C to 8°C (36°F to 46°F).
- Upon receipt, store HEMGENIX vials in a refrigerator at 2°C to 8°C (36°F to 46°F).
- Store HEMGENIX in the original carton until use.
- Protect HEMGENIX from light until time of dilution and administration.
- Do NOT FREEZE.
After dilution
- Once diluted, store HEMGENIX in the infusion bag protected from light.
- Store diluted HEMGENIX in the infusion bag at 15°C to 25°C (59°F to 77°F).
- Infuse the diluted product within 24 hours after the dose preparation [see Dosage and Administration (2.2)].
17. Patient Counseling Information
Inform patients that:
- Pre-infusion blood tests will be necessary to look for Factor IX inhibitors. If these exist, the patient may not be a good candidate for HEMGENIX [see Dosage and Administration (2)].
- Prior to HEMGENIX treatment, a liver ultrasound and elastography will be performed. Patients found to have pre-existing risk factors for hepatocellular carcinoma will be monitored annually in the 5 years following infusion [see Warnings and Precautions (5.4)].
- Infusion reactions can occur. Patients will be monitored during and for at least 3 hours following administration. If a reaction occurs, the infusion rate may be slowed or interrupted, then started at a slower rate [see Warnings and Precautions (5.1)].
- HEMGENIX can elevate certain liver enzymes. Weekly blood tests will be required to monitor for this for 3 months after treatment. Corticosteroid treatment may be necessary if this occurs [see Warnings and Precautions (5.2)].
- If post-infusion bleeding is not controlled or if bleeding returns, then blood tests will be performed for Factor IX activity and neutralizing Factor IX inhibitors [see Warnings and Precautions (5.5)].
- Vector distribution in blood (within the body), and vector shedding in semen and other excreta and secreta can occur post-infusion. It is not known how long this will continue. Patients should not donate blood, organs, tissues, or cells for transplantation [see Pharmacokinetics (12.3)].
Manufactured by:
uniQure, Inc.
113 Hartwell Avenue
Lexington, MA 02421 USA
Manufactured for:
CSL Behring LLC
King of Prussia, PA 19406, USA
US License No. 1767
Distributed by:
CSL Behring LLC
Kankakee, IL 60901 USA
For Patent information: www.cslbehring.com/products/patents (in-licensed from uniQure)
PRINCIPAL DISPLAY PANEL - 10 ml Vial Label
NDC 0053-0099-01
10 ml
etranacogene dezaparvovec-drlb
HEMGENIX®
1x1013 genome copies/mL
Suspension for intravenous infusion
Single-dose vial
Do Not Shake
Dilute before use
Rx only
CSL Behring
PRINCIPAL DISPLAY PANEL - 10 ml Vial Carton
NDC 0053-0099-01
etranacogene dezaparvovec-drlb
HEMGENIX®
1x1013 genome copies/mL
Suspension for intravenous infusion
Single-dose vial
1 x 1013 genome copies/mL.
No US standard of potency.
Upon receipt store refrigerated at
2°C to 8°C (36°F to 46°F)
Do Not Freeze
Protect from Light
Do Not Shake
Dilute before use
See enclosed prescribing information
for dosage and directions for use
Rx only
CSL Behring
PRINCIPAL DISPLAY PANEL - Kit Carton
etranacogene dezaparvovec-drlb
HEMGENIX®
1x1013 genome copies/mL
Suspension for intravenous infusion
Single-dose vial
Rx only
Upon receipt store refrigerated at 2°C to 8°C (36°F to 46°F)
Do Not Freeze
Protect from Light
Do Not Shake
Dilute before use
Manufactured by:
uniQure, Inc.
Lexington, MA 02421 USA
Manufactured for:
CSL Behring LLC
King of Prussia, PA 19406 USA
US License No. 1767
Distributed by:
CSL Behring LLC
Kankakee, IL 60901 USA
CSL Behring
PRINCIPAL DISPLAY PANEL - NDC: 0053-0100-10_ 46-50 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 46-50 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0110-11_ 51-55 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 51-55 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0120-12_ 56-60 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 56-60 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0130-13_ 61-65 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 61-65 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0140-14_ 66-70 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 66-70 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0150-15_ 71-75 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 71-75 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0160-16_ 76-80 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 76-80 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0170-17_ 81-85 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 81-85 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0180-18_ 86-90 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 86-90 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0190-19_ 91-95 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 91-95 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0200-20_ 96-100 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 96-100 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0210-21_ 101-105 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 101-105 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0220-22_ 106-110 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 106-110 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0230-23_ 111-115 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 111-115 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0240-24_ 116-120 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 116-120 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0250-25_ 121-125 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 121-125 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0260-26_ 126-130 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 126-130 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0270-27_ 131-135 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 131-135 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0280-28_ 136-140 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 136-140 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0290-29_ 140-145 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 140-145 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0300-30_ 146-150 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 146-150 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0310-31_ 151-155 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 151-155 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0320-32_ 156-160 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 156-160 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0330-33_ 161-165 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 161-165 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0340-34_ 166-170 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 166-170 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0350-35_ 171-175 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 171-175 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0360-36_ 176-180 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 176-180 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0370-37_ 181-185 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 181-185 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0380-38_ 186-190 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 186-190 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0390-39_ 191-195 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 191-195 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0400-40_ 196-200 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 196-200 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0410-41_ 201-205 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 201-205 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0420-42_ 206-210 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 206-210 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0430-43_ 211-215 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 211-215 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0440-44_ 216-220 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 216-220 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0450-45_ 221-225 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 221-225 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0460-46_ 226-230 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 226-230 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0470-47_ 231-235 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 231-235 kg
PRINCIPAL DISPLAY PANEL - NDC: 0053-0480-48_ 236-240 KG KIT VARIABLE LABEL
etranacogene dezaparvovec-drlb
HEMGENIX®
Patient Weight: 236-240 kg