Addamel N: Package Insert / Prescribing Info
Package insert / product label
Generic name: chromic chloride, cuprous chloride, ferric chloride, manganese chloride, potassium iodide, sodium fluoride, sodium molybdate dihydrate, sodium selenite and zinc chloride
Dosage form: injection, solution
Drug class: Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Mar 24, 2025.
On This Page
Medication Guide
1. QUALITATIVE AND QUANTITATIVE COMPOSITION
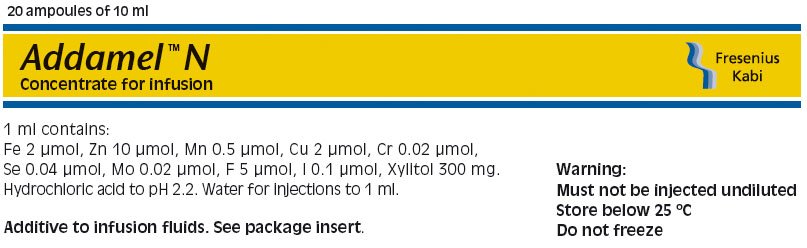
1 ml of ADDAMEL N contains:
Active ingredients Quantity
Chromic chloride 6 H2O 5.33 µg
Copper chloride 2 H2O 0.34 mg
Ferric chloride 6 H2O 0.54 mg
Manganese chloride 4 H2O 99.0 µg
Potassium iodide 16.6 µg
Sodium fluoride 0.21 mg
Sodium molybdate 2 H2O 4.85 µg
Sodium selenite anhydrous 6.90 µg
Zinc chloride 1.36 mg
The active ingredients in 1 ml of ADDAMEL N correspond to:
Cr 0.02 µmol
Cu 2 µmol
Fe 2 µmol
Mn 0.5 µmol
I 0.1 µmol
F 5 µmol
Mo 0.02 µmol
Se 0.04 µmol
Zn 10 µmol
The content of sodium and potassium correspond to
Sodium 118 µg 5.12 µmol
Potassium 3.9 µg 0.1 µmol
PRODUCT PROPERTIES
• Osmolality: approx. 3100 mosm/kg water
• pH: 2.2
2. PHARMACEUTICAL FORM
Concentrate for solution for infusion
3. CLINICAL PARTICULARS
3.1 Therapeutic indications
ADDAMEL N is indicated in patients as a supplement in intravenous nutrition to meet basal to moderately increased requirements of trace elements.
3.2 Posology and method of administration
ADDAMEL N must not be given undiluted.
The recommended daily dosage of ADDAMEL N in adult patients with basal to moderately increased requirements is 10 ml (one ampoule).
For children weighing 15 kg or more, the recommended dosage is 0.1 ml ADDAMEL N/kg body weight/day.
3.3 Contraindications
Total biliary obstruction.
3.4 Special warnings and special precautions for use
ADDAMEL N should be used with caution in patients with impaired biliary and/or renal function in whom the excretion of trace elements may be significantly decreased.
ADDAMEL N should also be used with caution in patients with biochemical or clinical evidence of liver dysfunction (especially cholestasis).
If the treatment is continued for more than 4 weeks, checking of manganese levels is required.
ADDAMEL N must not be given undiluted.
3.5 Interaction with other medicaments and other forms of interaction
No interactions with other drugs have been observed.
3.6 Pregnancy and lactation
Animal reproduction studies or clinical investigations during pregnancy have not been carried out with ADDAMEL N. However, the requirements of trace elements in a pregnant woman are slightly
increased compared to non-pregnant women.
No adverse events are to be expected when ADDAMEL N is administered during pregnancy.
3.7 Effects on ability to drive and use machines
No effects on the ability to drive and use machines are to be expected.
3.8 Undesirable effects
No adverse effects related to the trace elements in ADDAMEL N have been reported.
Superficial thrombophlebitis has been observed when glucose containing ADDAMEL N was given. However, it is not possible to deduce whether this reaction is attributable to the infused trace elements
or not.
Allergic reactions to iodine may occur following topical application. No adverse reactions are known to occur as a consequence of using the recommended intravenous iodide dosage levels.
3.9 Overdose
In patients with impaired renal or biliary function, there is an increased risk for accumulation of trace elements.
In case of a chronic overload of iron there is a risk of haemosiderosis, which in severe and rare cases can be treated by venesection.
4. PHARMACOLOGICAL PROPERTIES
4.1 Pharmacodynamic properties
ADDAMEL N is a mixture of trace elements in amounts normally absorbed from the oral diet and should have no pharmacodynamic effect besides maintaining or repleting the nutritional status.
4.2 Pharmacokinetic properties
When infused intravenously, the trace elements in ADDAMEL N are handled in a similar way to trace elements from an oral diet. Individual trace elements will be taken up by tissues to different extents,
depending on the requirements within each tissue to maintain or restore the concentration of each element for the metabolic requirements of that tissue.
Copper and manganese are normally excreted via the bile, whereas selenium, zinc and chromium (especially in patients receiving intravenous nutrition) are mainly excreted via the urine.
The main route of molybdenum excretion is the urine, although small amounts are excreted in the bile.
Iron is eliminated in small amounts by superficial loss and desquamation of gut cells. Premenopausal women can lose 30-150 mg of iron in the monthly blood loss. Iron excretion follows all kinds of
bleedings.
4.3 Preclinical safety data
The safety evaluation is based mainly on clinical experience and documentation.
5. PHARMACEUTICAL PARTICULARS
5.1 List of excipients
Other ingredients Quantity Reference to standards
Xylitol 300 mg Ph. Eur. + USP
Hydrochloric acid 1 M to pH 2.2 Ph. Eur.
Water for injections to 1 ml Ph. Eur.
5.2 Incompatibilities
ADDAMEL N may only be added to or mixed with other medicinal products for which compatibility has been documented. See 5.6.
5.3 Shelf life
24 months
5.4 Special precautions for storage
Store below 25°C. Do not freeze.
5.5 Nature and contents of container
Ampoule, polypropylene
Pack size: 20 x 10 ml
5.6 Instructions for use/handling
ADDAMEL N must not be given undiluted.
COMPATIBILITY
Additions should be made aseptically.
Up to 20 ml ADDAMEL N can be added to 1000 ml Vamin Glucose, Vamin 14 Electrolyte Free, Vamin 18 Electrolyte Free and glucose solutions 50 mg/ml-500 mg/ml.
STABILITY
When additions are made to an infusion solution, the infusion should be completed within 24 hours from preparation to prevent microbiological contamination. The left over contents of opened
bottles/vials/ampoules should be discarded and not kept for later use.
| ADDAMEL N
chromium, copper, iron, manganese, iodine, fluorine, molybdenum, selenium, and zinc injection, solution |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Fresenius Kabi USA, LLC (608775388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi Norge AS | 731170932 | MANUFACTURE(63323-143) | |