ORA 10 Pill: white, round, 6mm
Generic Name: prednisolone
The pill with imprint ORA 10 (White, Round, 6mm) has been identified as Orapred ODT 10 mg and is used for Asthma, acute, Dermatitis, Bronchopulmonary Dysplasia, Bullous Pemphigoid, and Crohn's Disease, Active. It belongs to the drug class glucocorticoids and is not a controlled substance.
Images for ORA 10
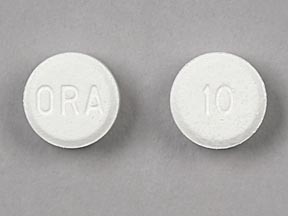
Orapred ODT
- Generic Name
- prednisolone
- Imprint
- ORA 10
- Strength
- 10 mg
- Color
- White
- Size
- 6.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Glucocorticoids
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Alliant Pharmaceuticals Inc
- National Drug Code (NDC)
- 68188-0480 (Discontinued)
Related images for "ORA 10"
More about Orapred ODT (prednisolone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: glucocorticoids
- Breastfeeding
- En español
Patient resources
Other brands
Prelone, PediaPred, Bubbli-Pred, Flo-Pred, ... +4 more
Professional resources
- Orapred ODT prescribing information
- PrednisoLONE, prednisoLONE Acetate, prednisoLONE Sodium Phosphate (AHFS Monograph)
Other brands
Orapred, PediaPred, Flo-Pred, Millipred, Veripred 20
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.