Methocarbamol FDA Alerts
The FDA Alerts below may be specifically about methocarbamol or relate to a group or class of drugs which include methocarbamol.
MedWatch Safety Alerts are distributed by the FDA and published by Drugs.com. Following is a list of possible medication recalls, market withdrawals, alerts and warnings.
Recent FDA Alerts for methocarbamol
Eugia US LLC Issues Voluntary Nationwide Recall of Methocarbamol Injection, USP 1000 mg/10 mL Due to Presence of White Particles
March 28, 2024– East Windsor, New Jersey, Eugia US LLC (f/k/a AuroMedics Pharma LLC) has initiated a voluntary recall of lot number 3MC23011 of Methocarbamol Injection, USP 1000 mg/10 mL (100mg/mL) (Single Dose Vial) - 10mL Vial to the consumer level due to a customer product complaint for the presence of white particles floating inside of the vial.
Risk Statement: Administration of an injectable product that contains particulate matter may result in local irritation or swelling. If the particulate matter reaches the blood vessels or is injected intravascularly it can travel to various organs and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death. To date, Eugia US LLC has not received any reports of adverse events related to this recall.
Methocarbamol injection USP 1000 mg/10 mL (100mg/mL), is used as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. It is packaged in 10 mL and packed as 25 (vials) X 04 (Printed E-Flute cartons) X 01 (Shipper) with NDC code as 55150-223-10. Eugia US LLC shipped the entire lot to wholesalers nationwide from Jan 12, 2024, through Jan 16, 2024.
The product can be identified by product name on carton and vial label and with lot number 3MC23011 and Exp. Date: Nov 2026 (NDC 55150-223-10).
Eugia US LLC (f/k/a AuroMedics Pharma LLC) is notifying its distributors by recall letters and is arranging for the return/replacement of all recalled products. Wholesalers, hospitals, pharmacies, institutions, and doctors with an existing inventory of the recalled product lot should discontinue use, stop distribution and quarantine the product immediately. If you have further distributed the recalled product lot, notify your accounts and/or any additional locations which may have received the recalled product. Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall.
Consumers with medical questions regarding this recall or to report an adverse event can contact Eugia US LLC from 8:00 am to 5:00 pm (EST) Monday - Friday at:
- 1-866-850-2876 Option 2
- pvg@aurobindousa.com
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
If you have any general questions regarding the return of this product, please contact Qualanex at 1-800-505-9291 or email recall@qualanex.com (live calls received 7:00 am to 4:00 pm M-F CST).
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: FDA
FDA Warns Consumers Not to Purchase or Use Artri and Ortiga Products, Which May Contain Hidden Drug Ingredients
April 20, 2021 --FDA is warning consumers not to purchase or use products marketed with variations of the names “Artri” or “Ortiga” due to potentially dangerous hidden active drug ingredients not listed on the product label. FDA urges consumers taking these products to immediately talk to their health care professional (e.g., doctor) to safely discontinue use of the product because suddenly stopping these drugs may be dangerous.
These products are promoted for treating arthritis, muscle pain, osteoporosis, bone cancer, and other conditions and are sold on various websites and in some retail stores.
FDA laboratory analyses revealed certain Artri and Ortiga products contain the undeclared drug ingredients:
- Dexamethasone (a corticosteroid) that can cause serious adverse events, including infections, increased blood glucose (sugar) levels, changes in blood pressure, damage to bones, psychiatric problems, and adrenal dysfunction;
- Diclofenac sodium (an anti-inflammatory drug) that can lead to adverse cardiovascular events, such as heart attack and stroke, or serious gastrointestinal damage, including bleeding, ulceration, and fatal tears of the stomach and intestines, or liver toxicity including liver failure that can cause the need for a liver transplant or death
- Methocarbamol (a muscle relaxant) that can cause sedation, dizziness, and low blood pressure.
These drug ingredients, which are not listed on the product label, can also interact with other drugs a consumer is taking.
FDA has received adverse event reports, including of liver toxicity and death, associated with the use of Artri King products, since the agency issued its first warning about an Artri Ajo King product on January 5, 2022.
Suddenly stopping corticosteroids after long-term use or high doses can result in a serious withdrawal syndrome that includes fatigue, nausea, low blood pressure, low blood glucose levels, fever, dizziness, muscle and joint pain, and shortness of breath. These risks depend on several factors that a health care professional must assess. Medical intervention may be necessary.
Health care professionals should evaluate patients who have used Artri and Ortiga products for drug and disease interactions involving diclofenac, methocarbamol, and corticosteroids, and treat accordingly.
FDA has identified the following Artri and Ortiga products containing hidden drug ingredients:
FDA analyses reflect only the undeclared ingredients discovered in one product from a specific lot, but ingredients may vary from product to product or from lot to lot. Products marketed as dietary supplements that are found to have hidden drug ingredients generally fail to comply with most current good manufacturing practices designed to ensure product quality and safety. Therefore, consumers should expect the manufacturing processes for Artri and Ortiga products are unreliable in providing consistent amounts of active ingredients or to prevent the introduction of unknown chemicals or other impurities.
FDA is investigating the distribution of these products in the United States and has advised certain companies not to sell or distribute these products. The agency may take additional enforcement steps that may include warning letters, seizure, injunction, or criminal charges.
Health care professionals and consumers should report adverse events or side effects related to the use of this product to FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report online at www.fda.gov/medwatch/report.htm; or
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Source: FDA
Bryant Ranch Prepack Issues Voluntary Nationwide Recall of Methocarbamol 500mg Bottles Due to Mislabeling With the Incorrect Strength
October 19, 2021 -- Burbank, CA, Bryant Ranch Prepack is voluntarily recalling 1 lot of Methocarbamol 500mg, Tablets to the consumer level. The bottles labeled as Methocarbamol 500mg Tablets have been found to contain Methocarbamol 750mg Tablets.
Risk Statement: If a patient takes a 750mg Tablet of Methocarbamol instead of the prescribed 500mg Tablets, it potentially could result in Excessive Central Nervous System depression which may result in nausea, sedation, fainting, falls, seizure, coma, and death. Bryant Ranch Prepack has not received any reports of adverse events related to this recall.
The product is used together with rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions.
The product is packaged in a white round bottle with a red and white label, which reads Methocarbamol 500mg packaged in counts of 30 (NDC:7133517952), 60 (NDC: 7133517954), and 90 (NDC:7133517957) pills. The affected Methocarbamol 500mg lots include the following Lot Number 163935/ Exp. Date 10/22. The product can be identified by red and white label with a yellow border at the top and bottom of the label, top of the label reads “Packaged by Bryant Ranch Prepack”, labels are pictured here. The Methocarbamol 500mg was distributed Nationwide to multiple physician offices.
Bryant Ranch Prepack is notifying its distributors and customers by letter and email and is arranging for return of all recalled products. Consumers that have the bottles labeled as Methocarbamol 500mg Tablets which is being recalled should stop using immediately and return to place of purchase and/or contact their physician. Distributors/Physicians should stop distribution and contact Bryant Ranch Prepack to return the product immediately.
Consumers with questions regarding this recall can contact Bryant Ranch Prepack by phone at 877-885-0882 Mon.-Fri. 7am-6pm PST or compliance@brppharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: FDA
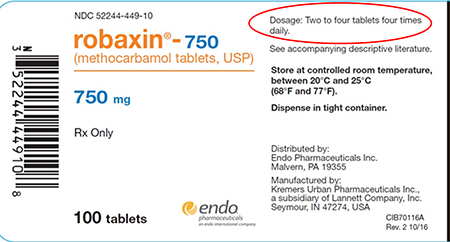
Endo Pharmaceuticals Issues Voluntary Nationwide Recall for Two Lots of Robaxin 750mg Tablets 100 Count Bottle Packs Due to Incorrect Daily Dosing Information on Label
Endo International plc (NASDAQ: ENDP) today announced that one of its operating companies, Endo Pharmaceuticals Inc., is voluntarily recalling two lots of Robaxin® (methocarbamol tablets, USP) 750mg Tablets 100 Count Bottle pack to the consumer level. The products have been found to have incorrect daily dosing information on the label due to a labeling error which misstates the daily dose as "two to four tablets four times daily" rather than the correct dosage of "two tablets three times daily." (see picture below for location of incorrect text).
Patients who follow the directions on the bottle may experience significant drowsiness or dizziness which would put them at risk of falls or an overdose which could result in seizures, coma, or death. To date, Endo Pharmaceuticals Inc. has not received any reports of adverse events related to this recall.
Robaxin® 750mg Tablets contain the active ingredient methocarbamol and are indicated as an adjunct therapy to rest, physical therapy and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. Robaxin® 750mg Tablets are packed in bottles of 100 tablets with package labeling featuring the product name, strength, lot number, expiry date and the National Drug Code number NDC 52244-449-10.
The recall includes the following product lots:
- Robaxin® 750mg, 100 Count Bottle pack, Lot 216702P1, Expiration Date: September 2020; and
- Robaxin® 750mg, 100 Count Bottle pack, Lot 220409P1, Expiration Date: January 2021.
No other lots of Robaxin® are affected by this market action.
Robaxin® 750mg 100 Count Bottle packs were distributed by wholesale distributors to retail pharmacies.
Endo Pharmaceuticals Inc. is notifying distributors and retailers in writing through Inmar, Inc. Inmar is arranging for return of all recalled products.
Distributors and retailers that have product which is being recalled should stop distributing and dispensing and return to the place of purchase.
Consumers in possession of any unused prescribed Robaxin® 750mg product bearing lot numbers 216702P1 or 220409P1 should discontinue use of the product and return the unused product by following the instructions below:
- Please contact Inmar at 1-866-391-0620, Monday through Friday (9am to 5pm ET) or email robaxin@inmar.com for the following:
- Product Return
- Upon contacting Inmar and indicating you have unused product, please expect Return Authorization labels and Shipping instructions.
- Product Reimbursement
- Upon contacting Inmar, please be prepared to share proof of purchase.
- Proof of purchase can be sent to robaxin@inmar.com or 635 Vine St.Winston Salem, NC 27101-Attention Recall Department, Robaxin Recall.
- Upon contacting Inmar, please be prepared to share proof of purchase.
- Product Return
Distributors, retailers and consumers with questions regarding this recall can contact Inmar by telephone at 1-866-391-0620 during the following hours: Monday through Friday (9am to 5pm ET) or by email at robaxin@inmar.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax.
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This Product Recall is being made with the knowledge of the United States Food and Drug Administration (FDA).
Endo Pharmaceuticals Inc. takes this issue seriously and works to achieve high quality standards for all of its products and packaging. If you have any questions, please call 1-800-462-ENDO (3636), between the hours of 8:00 a.m. to 8:00 p.m. ET Monday through Thursday and 8:00 a.m. to 6:00 p.m. ET on Friday.
Reumofan Plus: Recall - Undeclared Drug Ingredient
Reumofan Plus USA, LLC and Reumofan USA, LLC is recalling "Reumofan Plus" Tablets, Lot# 99515, exp. 09/16, because they contain undeclared active pharmaceutical ingredients: methocarbamol, dexamethasone, and diclofenac. The recall was initiated after it was discovered that the product was distributed in packaging that did not reveal the presence of the active pharmaceutical ingredients, making it an unapproved drug. One illness has been reported to date in connection with this problem.
[UPDATED 8/28/2012]
Samantha Lynn Inc. is voluntarily recalling 500 lots of Reumofan Plus Tablets to the consumer level due to findings of undeclared drug ingredients. The FDA sample analysis has found the product to contain methocarbamol and diclofenac. The affected Reumofan Plus lots may include the following lot number(s): 99515 ex096 and expires: 2016. The product is marketed in a green bottle containing 30 lavender round tablets and is distributed nationwide via the internet.
[UPDATED 08/21/2012]
FDA is issuing an updated alert that Reumofan Plus and Reumofan Plus Premium contain undeclared active ingredients found in prescription drugs that should be used only under the supervision of a health care professional.
Since June 1, 2012, when FDA first warned the public about the dangers of these supplements, the agency has received reports of fatalities, stroke, severe bleeding in the gastrointestinal tract, dizziness, insomnia, high blood sugar levels and problems with liver and kidney functions,as well as corticosteroid withdrawal syndrome
Because of the possible risks, consumers should not buy or start using these products.
[Posted 06/01/2012]
ISSUE: FDA is warning consumers that Reumofan Plus, marketed as a natural dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful. An FDA laboratory analysis of Reumofan Plus found that it contains Diclofenac Sodium, a prescription non-steroidal anti-inflammatory drug (NSAID) that may cause increased risk of cardiovascular events such as heart attack and stroke, as well as serious gastrointestinal (GI) adverse events including bleeding, ulceration, and fatal perforation (causing a hole) of the stomach and intestines, and Methocarbamol, a prescription muscle relaxant that can cause sedation, dizziness, low blood pressure, and impair mental or physical abilities to perform tasks such as driving a motor vehicle or operating machinery.
The Mexican Ministry of Health discovered that at least one lot of the product contains the corticosteroid dexamethasone, a drug that acts as an anti-inflammatory and immune system suppressant.
FDA has received multiple reports of adverse events associated with the use of Reumofan Plus, including liver injury, sudden worsening of glucose control, weight gain, swelling, leg cramps, and adrenal suppression.
BACKGROUND: Reumofan Plus is marketed as a natural dietary supplement for pain relief. Reumofan Plus is labeled in Spanish and promoted for treating arthritis, muscle pain, osteoporosis, bone cancer, and other conditions. The product is manufactured in Mexico by Riger Naturals and sold in some retail outlets, at flea markets, and on various internet sites. FDA has worked closely with the Mexican government on this matter. The Mexican Ministry of Health has issued a health warning to the public and ordered Riger Naturals to recall the product.
RECOMMENDATION: Consumers who are currently taking or who recently stopped taking Reumofan Plus are urged to consult a healthcare professional immediately. Health care professionals are urged to ask their patients about use of Reumofan Plus and other products marketed as dietary supplements when patients present with unexplained symptoms that suggest NSAID toxicity, depression, or the use or abrupt discontinuation of corticosteroids. Additionally, health care professionals should evaluate patients who have used Reumofan Plus for drug and disease interactions involving diclofenac, methocarbamol, and corticosteroids, and consider whether a corticosteroid taper regimen may be appropriate in those who have used Reumofan Plus.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[08/21/2012 - News Release - FDA]
[08/21/2012 - Consumer Update - FDA]
[06/01/2012 - News Release - FDA]
Reumofan Plus Dietary Supplement Relabeled and Sold as “WOW”: Public Warning - Undeclared Drug Ingredients
ISSUE: The U.S. Food and Drug Administration (FDA) is warning the public that the potentially harmful dietary supplement product Reumofan Plus is being relabeled and sold under the name “WOW.” The product is being marketed to treat arthritis, muscle pain, osteoporosis, bone cancer, and other conditions. FDA laboratory analysis confirmed that “WOW” contains the same prescription drug ingredients that are in Reumofan Plus, including dexamethasone (a corticosteroid), diclofenac sodium (a non-steroidal anti-inflammatory drug), and methocarbamol (a muscle relaxant). These ingredients have the potential to cause serious injury.
BACKGROUND: FDA warned the public of the harm of Reumofan Plus on June 1, 2012, and again on August 21, 2012. Since June, FDA has received dozens of adverse event reports, many of them serious, from consumers who used Reumofan Plus. The reports include liver injury, severe bleeding, corticosteroid withdrawal syndrome, adrenal suppression, stroke, and even death.
Reumofan Plus and “WOW” products are sold on various websites, including Gonepainfree.com and Browerent.com. The products are manufactured by Riger Naturals S.A. In addition to websites selling “WOW,” FDA has become aware that various websites, including Reumofanusa.com, owned by Reumofan USA, LLC, continue to sell Reumofan Plus even after previous FDA warnings. Please see the link to the FDA public warning for product photos.
RECOMMENDATION: Consumers currently taking or who have taken Reumofan Plus or “WOW” should immediately consult a health care professional. Health care professionals are urged to ask their patients about the use of Reumofan Plus, “WOW,” and other similar products marketed as dietary supplements when patients present with unexplained symptoms that suggest NSAID toxicity, psychiatric changes, or the use or abrupt discontinuation of corticosteroids.
Additionally, health care professionals should evaluate patients who have used Reumofan Plus and/or WOW for drug and disease interactions involving diclofenac, methocarbamol, and corticosteroids, and consider whether a corticosteroid taper regimen may be appropriate.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of Reumofan Plus products and “WOW” to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
[12/19/2012 - FDA Public Warning - FDA]
Previous Related Safety Alert:
[08/21/2012 - Reumofan Plus MedWatch Safety Alert - FDA]
Reumofan Plus: Recall - Undeclared Drug Ingredient
[UPDATED 08/21/2012]
FDA is issuing an updated alert that Reumofan Plus and Reumofan Plus Premium contain undeclared active ingredients found in prescription drugs that should be used only under the supervision of a health care professional.
Since June 1, 2012, when FDA first warned the public about the dangers of these supplements, the agency has received reports of fatalities, stroke, severe bleeding in the gastrointestinal tract, dizziness, insomnia, high blood sugar levels and problems with liver and kidney functions,as well as corticosteroid withdrawal syndrome
Because of the possible risks, consumers should not buy or start using these products.
[Posted 06/01/2012]
ISSUE: FDA is warning consumers that Reumofan Plus, marketed as a natural dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful. An FDA laboratory analysis of Reumofan Plus found that it contains Diclofenac Sodium, a prescription non-steroidal anti-inflammatory drug (NSAID) that may cause increased risk of cardiovascular events such as heart attack and stroke, as well as serious gastrointestinal (GI) adverse events including bleeding, ulceration, and fatal perforation (causing a hole) of the stomach and intestines, and Methocarbamol, a prescription muscle relaxant that can cause sedation, dizziness, low blood pressure, and impair mental or physical abilities to perform tasks such as driving a motor vehicle or operating machinery.
The Mexican Ministry of Health discovered that at least one lot of the product contains the corticosteroid dexamethasone, a drug that acts as an anti-inflammatory and immune system suppressant.
FDA has received multiple reports of adverse events associated with the use of Reumofan Plus, including liver injury, sudden worsening of glucose control, weight gain, swelling, leg cramps, and adrenal suppression.
BACKGROUND: Reumofan Plus is marketed as a natural dietary supplement for pain relief. Reumofan Plus is labeled in Spanish and promoted for treating arthritis, muscle pain, osteoporosis, bone cancer, and other conditions. The product is manufactured in Mexico by Riger Naturals and sold in some retail outlets, at flea markets, and on various internet sites. FDA has worked closely with the Mexican government on this matter. The Mexican Ministry of Health has issued a health warning to the public and ordered Riger Naturals to recall the product.
RECOMMENDATION: Consumers who are currently taking or who recently stopped taking Reumofan Plus are urged to consult a healthcare professional immediately. Health care professionals are urged to ask their patients about use of Reumofan Plus and other products marketed as dietary supplements when patients present with unexplained symptoms that suggest NSAID toxicity, depression, or the use or abrupt discontinuation of corticosteroids. Additionally, health care professionals should evaluate patients who have used Reumofan Plus for drug and disease interactions involving diclofenac, methocarbamol, and corticosteroids, and consider whether a corticosteroid taper regimen may be appropriate in those who have used Reumofan Plus.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[08/21/2012 - News Release - FDA]
[08/21/2012 - Consumer Update - FDA]
[06/01/2012 - News Release - FDA]
Reumofan Plus: Recall - Undeclared Drug Ingredient
ISSUE: FDA is warning consumers that Reumofan Plus, marketed as a natural dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful. An FDA laboratory analysis of Reumofan Plus found that it contains Diclofenac Sodium, a prescription non-steroidal anti-inflammatory drug (NSAID) that may cause increased risk of cardiovascular events such as heart attack and stroke, as well as serious gastrointestinal (GI) adverse events including bleeding, ulceration, and fatal perforation (causing a hole) of the stomach and intestines, and Methocarbamol, a prescription muscle relaxant that can cause sedation, dizziness, low blood pressure, and impair mental or physical abilities to perform tasks such as driving a motor vehicle or operating machinery.
The Mexican Ministry of Health discovered that at least one lot of the product contains the corticosteroid dexamethasone, a drug that acts as an anti-inflammatory and immune system suppressant.
FDA has received multiple reports of adverse events associated with the use of Reumofan Plus, including liver injury, sudden worsening of glucose control, weight gain, swelling, leg cramps, and adrenal suppression.
BACKGROUND: Reumofan Plus is marketed as a natural dietary supplement for pain relief. Reumofan Plus is labeled in Spanish and promoted for treating arthritis, muscle pain, osteoporosis, bone cancer, and other conditions. The product is manufactured in Mexico by Riger Naturals and sold in some retail outlets, at flea markets, and on various internet sites. FDA has worked closely with the Mexican government on this matter. The Mexican Ministry of Health has issued a health warning to the public and ordered Riger Naturals to recall the product.
RECOMMENDATION: Consumers who are currently taking or who recently stopped taking Reumofan Plus are urged to consult a healthcare professional immediately. Health care professionals are urged to ask their patients about use of Reumofan Plus and other products marketed as dietary supplements when patients present with unexplained symptoms that suggest NSAID toxicity, depression, or the use or abrupt discontinuation of corticosteroids. Additionally, health care professionals should evaluate patients who have used Reumofan Plus for drug and disease interactions involving diclofenac, methocarbamol, and corticosteroids, and consider whether a corticosteroid taper regimen may be appropriate in those who have used Reumofan Plus.
Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.accessdata.fda.gov/scripts/medwatch/index.cfm
- Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
[06/01/2012 - News Release - FDA]
