Zactran Injectable Solution (Canada)
This treatment applies to the following species: Company: Merial
Company: Merial
(gamithromycin)
Veterinary Use Only
DIN 02347407
Description
ZACTRAN® Injectable Solution is a ready-to-use sterile parenteral solution containing gamithromycin, a macrolide sub-class, 7a-azalide antimicrobial. Each mL of ZACTRAN Injectable Solution contains 150 mg of gamithromycin, 40 mg of succinic acid, 1 mg of monothioglycerol and glycerol formal q.s. ad 1 mL.
The chemical name of gamithromycin is 1-Oxa-7-azacydopentadecan-15-one,13-[(2,6-dideoxy-3-C-methyl-3-0-methyl -.alpha.-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy 3,5,8,10,12,14 - hexamethyl - 7 - propyl - 11 - {[3,4,6 - trideoxy - 3 - (dimethylamina) - .beta. - D - xylo - hexopyranosyl]oxy} - ,[(2R*,3S*,4R*,5S*,8R*,10R*,11R*,12S*,13S*, 14R*)]-and the structure is shown below.
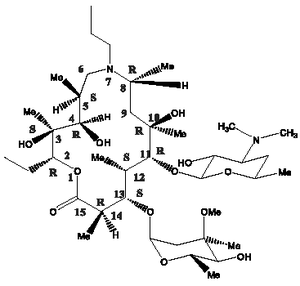
Zactran Injectable Solution Indications
ZACTRAN Injectable Solution is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni (Haemophilus somnus) and Mycoplasma bovis. ZACTRAN Injectable Solution is also indicated for the reduction of morbidity associated with BRD in feedlot calves, caused by Mannheimia haemolytica, Pasteurella multocida and Histophilus somni, during the first 10 days in the feedlot, when administered at the time of arrival.
Dosage and Administration
Administer a single subcutaneous injection in the neck at a dose of 6 mg gamithromycin/kg body weight (equivalent to 1 mL/25 kg body weight).
For treatment of cattle over 250 kg body weight, divide the dose so that no more than 10 mL are injected at one site.
|
Body Weight (kg) |
Dose Volume (mL) |
|
50 |
2 |
|
100 |
4 |
|
200 |
8 |
|
300 |
12 |
|
400 |
16 |
|
500 |
20 |
Most animals will respond to treatment within 3 to 5 days. If no improvement is observed, the diagnosis should be re-evaluated.
Note: To limit the development of antimicrobial resistance, ZACTRAN Injectable Solution should only be used as an arrival treatment when: 1) BRD has been diagnosed and 2) calves are at «high risk» of developing BRD. One or more of the following factors typically characterizes calves at «high risk» of developing BRD. Cattle are from multiple farm origins, and/or cattle have extended transport times (that may have included few if any rest stops), and/or ambient temperature change(s) from origin to arrival of 17°C or more, and/or cattle have experienced excessive shrink or stressful processing procedures such as castration and dehorning.
Clinical Pharmacology
The macrolide antimicrobials as a class are weak bases and as such concentrate in bronchoalveolar macrophages which are relevant to the successful treatment and control of BRD. Macrolides, in general, have been shown to have an extended post-antibiotic effect, which may also support the extended antimicrobial activity. Gamithromycin is primarily bacteriostatic at therapeutic concentrations. However, in vitro bactericidal activity has been observed for Mannheimia haemolytica and Pasteurella multocida at concentrations of 10 µg/mL (Mueller Hinton Broth) and for Mannheimia haemolytica after exposure to 6-hour plasma samples derived from cattle dosed at 6 mg gamithromycin/kg body weight.
ZACTRAN Injectable Solution administered subcutaneously in the neck of cattle at a single dosage of 6 mg/kg body weight is well absorbed and >98% bioavailable with no gender differences. The peak plasma gamithromycin concentration occurs within 30 to 60 min after injection. Based upon plasma and lung homogenate data, the terminal elimination half-life (T1/2) of gamithromycin is 2.3 days in plasma and 3.8 days in lungs. In vitro plasma protein binding studies show that 26% of the gamithromycin binds to plasma protein, resulting in free drug available for rapid and extensive distribution into body tissues. The free drug is rapidly cleared from the systemic circulation with a clearance rate of 712 mL/hr/Kg and a volume of distribution of 25 L/kg. Gamithromycin reached peak concentration in lung homogenates within 24 hours after dosing; indicating that the drug was absorbed rapidly into the target tissue for BRD; and remained 247-fold above that obtained in the plasma concentration for at least 15 days. However, because tissue homogenate data do not necessarily reflect the concentration of free active drug at the infection site, the clinical relevance of these lung homogenate concentrations is not known.
Biliary excretion of the unchanged drug is the major route of elimination.
MICROBIOLOGY:
Macrolides action is mediated through disruption of bacterial protein synthesis. Macrolides inhibit bacterial protein biosynthesis by binding to the 50S ribosomal subunit and by preventing peptide chain elongation.
In vitro activity of gamithromycin has been demonstrated against Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis, four major pathogens associated with BRD.
The Minimum Inhibitory Concentrations (MIC) of gamithromycin were determined for isolates obtained from animals enrolled in field studies in the United States (U.S.).
|
Organism |
MIC50 (µg/mL) |
MIC90 (µg/mL) |
No. of isolates |
|
M. haemolytica* |
1.0 |
2 |
142 |
|
P. multocida* |
0.5 |
1 |
144 |
|
H. somni* |
0.5 |
1 |
70 |
|
M. bovis |
4 |
4 |
37 |
The minimum inhibitory concentration of gamithromycin inhibited 50% and 90% of the isolates.
* Clinical isolates supported by clinical data and indications for use.
EFFICACY:
The effectiveness of ZACTRAN Injectable Solution for the treatment of BRD was demonstrated at four geographic locations in the U.S. A total of 497 calves exhibiting clinical signs of BRD were enrolled in the study. Three hundred and thirty one calves were administered ZACTRAN Injectable Solution (6 mg/kg body weight) and 166 were administered an equivalent volume of sterile saline as a subcutaneous injection once on Day 0. Success was achieved based on rectal temperature below 40°C, and no clinically relevant evidence of depression or abnormal respiratory character an Day 10. The percentage of successes in cattle treated with ZACTRAN Injectable Solution (64%) was statistically significantly higher (p<0.05) than the percentage of successes in the cattle treated with saline (22%). There were two BRD-related deaths in the 331 ZACTRAN Injectable Solution treated calves compared to 14 BRD-related deaths in the 166 saline-treated calves.
The effectiveness of ZACTRAN Injectable Solution for the reduction of morbidity associated with BRD was demonstrated by two independent studies in the U.S. A total of 467 cattle at high risk were enrolled in the study. A total of 260 calves were administered ZACTRAN Injectable Solution (6 mg/kg body weight) and 207 were administered an equivalent volume of sterile saline as a subcutaneous injection once within the next day after arrival. Success was achieved based on rectal temperature below 40°C, and no clinically relevant evidence of depression or abnormal respiratory character on Day 10. The percentages of successes in the cattle treated with ZACTRAN Injectable Solution (86%, 78%) was statistically significantly higher (P<0.002) than the percentage of successes in the cattle treated with saline (36%, 58%).
An experimentally induced infection model study was designed to establish the efficacy of ZACTRAN Injectable Solution in the treatment of Mycoplasma bovis associated BRD. The study used an M. bovis isolate cultured from feedlot cattle. Calves exhibiting evidence of respiratory disease after challenge were enrolled and randomly assigned to ZACTRAN Injectable Solution and Saline treated groups. The lung lesions in the ZACTRAN Injectable Solution treated group were statistically significantly lower (p=0.024) where the mean percent total lung consolidation score for the Saline contra) was 14.0% compared with the ZACTRAN Injectable Solution mean of 4.63%. Lung lesions identified at necropsy were cultured for M. bovis. Some of the ZACTRAN Injectable Solution treated animals had lung lesions that were culture positive far M. bovis. The clinical significance of this finding, as it relates to potential relapses and/or persistent subclinical infections, is unknown.
ANIMAL SAFETY:
In a target animal safety study in six-month old beef calves, ZACTRAN Injectable Solution was administered by subcutaneous injection at 6, 18 and 30 mg/kg body weight (1, 3 and 5 times the labeled dose) on day 0, 5 and 10. Animals were clinically evaluated up to Day 15.
In overdosed groups, transient indications of pain after injection were seen, including head twists, pawing at the ground or attempts to lick the injection sites.
Dose related swellings characterized by skin thickening and discolouration of subcutaneous tissue were observed at the injection site, as well as related microscopic changes.
No other clinically significant drug-related effects were observed.
Warnings
Treated cattle must not be slaughtered for use in food for at least 49 days after the latest treatment with this drug. Do not use in lactating dairy cattle. Do not use in gestating cows or heifers, which are intended to produce milk for human consumption, within 2 months of expected parturition. To limit the development of antimicrobial resistance, ZACTRAN Injectable Solution should only be used as an arrival treatment in feedlot calves when BRD has been diagnosed and calves are at high risk of developing BRD.
Not for use in humans. Keep out of reach of children. Avoid contact with skin or eyes. If eye exposure occurs, flush eyes immediately with clean water. If skin exposure occurs, wash the affected area immediately with clean water. Consult a physician in case of accidental injection or ingestion by humans. Wash hands after use.
Zactran Injectable Solution Caution
The effects of ZACTRAN Injectable Solution on reproductive performance, pregnancy and lactation have not been determined.
Subcutaneous injection can cause a local tissue reaction that may result in trim loss of edible tissue at slaughter.
Contraindications
Do not use in case of hypersensitivity to macrolide drugs. Do not use this product simultaneously with other macrolides or antibiotics known as lincosamides.
Adverse Reactions
Transient injection site swellings with occasional slight pain have been observed in some animals.
No systemic adverse drug reactions were observed during clinical field studies.
Storage
Store at 15 - 30°C. Do not freeze. Discard all unused portions 28 days after opening the vial.
PRESENTATION:
ZACTRAN Injectable Solution is available in multidose 100 mL, 250 mL and 500 mL vials. Not all pack sizes maybe marketed.
Merial Canada Inc., 20000 Clark Graham, Baie d’Urfe, QC, H9X 4B6
© 2010 Merial Limited. AII Rights Reserved.
Canada Patents Nos. 2,064,634; 2,301,872 and 2,336,809
ZACTRAN® is a registered trademark of Merial Limited. The Cattle head logo is a registered trademark of Merial Limited, Duluth, GA 30096, US
Merial Limited, a company limited by shares registered in England and Wales (registered number 3332751) with a registered office at PO Box 327, Sandringham House, Sandringham Avenue, Harlow Business Park, Harlow, Essex CM19 5QA, England, and domesticated in Delaware, USA as Merial LLC, and having its place of business at 3239 Satellite Boulevard, Duluth, GA, U.S.A.
053 900877
CPN: 11821391
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
Elsewhere on our site
Qulipta
Qulipta is used to help prevent episodic or chronic migraine headaches in adults. Qulipta is an ...
Aimovig
Learn about Aimovig (erenumab-aooe) a once-monthly, injectable medication that can be ...
Dupixent
Dupixent is used to treat eczema, eosinophilic or oral-corticosteroid-dependent asthma, chronic ...
Ubrelvy
Ubrelvy (ubrogepant) tablets are used for the acute treatment of migraine. Includes Ubrelvy side ...
Nurtec ODT
Nurtec ODT (rimegepant) is used to treat acute migraines and prevent episodic migraines, by ...
Xeomin
Xeomin (incobotulinumtoxinA) is used to treat cervical dystonia, blepharospasm, upper facial lines ...
Dysport
Dysport (abobotulinumtoxinA) is used to treat cervical dystonia, glabellar lines and limb ...
Botox Cosmetic
Botox Cosmetic is a prescription treatment for fine lines and wrinkles. It temporarily improves the ...
