TiterCHEK CDV/CPV
This treatment applies to the following species:CANINE DISTEMPER-PARVOVIRUS ANTIBODY TEST KIT
TiterCHEK® CDV-CPV is an ELISA based assay used for the determination of antibody levels to Canine Distemper Virus (CDV) and Canine Parvovirus (CPV) in canine serum or plasma samples.
GENERAL INFORMATION AND INTENDED USES
Color coded plastic wells are coated with either purified canine distemper virus (CDV) antigen or canine parvovirus (CPV) antigen. Serum or plasma samples are incubated in the coated wells followed by incubation with polyclonal rabbit anti-dog lgG conjugated to horseradish peroxidase (HRP). Antibodies to CDV and/or CPV, if present in the canine samples, are bound to the specific antigen coated wells and, in turn, bind the anti-dog lgG conjugate. The free, unbound enzyme-linked conjugate is washed away and a chromogenic substrate is added to each well. A chromogenic color change (from clear to blue) occurs in the presence of the peroxidase enzyme. The blue color indicates a positive result. For CDV, a positive test result is intended to indicate a serum neutralization titer of 1:16 or greater, and a negative test result should indicate a serum neutralization titer of less than 1:16. For CPV, a positive test result is intended to indicate a hemagglutination inhibition (HI) titer of 1:80 or greater, and a negative test result should indicate a hemagglutination inhibition titer of less than 1:80.
TiterCHEK CDV-CPV is highly specific, sensitive and simple to perform. Test results can be obtained in 15 minutes. The diagnostic kit contains a positive control and a negative control which must be included each time the assay is performed. Visual comparison of the color of samples to the positive control for each assay will allow accurate detection of the presence of CDV and/or CPV antibody in the sample.
KIT COMPOSITION AND CONSERVATION
Contains materials sufficient to test 5 - 14 samples.
|
ITEM |
REAGENT NATURE |
VOLUME |
RECONSTITUTION AND CONSERVATION |
|
1 |
CDV Antigen Coated Wells |
2x8 wells |
Ready to use. CDV wells have a white rim. |
|
2 |
CPV Antigen Coated Wells |
2x8 wells |
Ready to use. CPV wells have a red rim. |
|
CONTROL + |
Positive Control; preserved with Phenol and Gentamicin sulfate |
1.0 mL |
Ready to use. (Red cap) |
|
B |
Negative Control/Sample Diluent; preserved with Phenol and Gentamicin sulfate |
3.0 mL |
Ready to use. (Gray cap) |
|
C |
HRP-Conjugate; preserved with Phenol and Gentamicin sulfate |
2.0 mL |
Ready to use. (Blue cap) |
|
D |
Chromogenic Substrate |
3.0 mL |
Ready to use. (Green cap) |
|
E |
10X Wash Concentrate |
100 mL |
Dilute to 1X in deionized or distilled water. (Orange cap) |
|
|
Disposable Sample Loops |
48 |
Ready to use |
|
|
Well Holder |
1 |
Ready to use |
Note: All reagents provided in the kit should be stored at 2 - 7 °C. Reagents should not be frozen.
EQUIPMENT AND MATERIALS REQUIRED BUT NOT PROVIDED
a) Deionized or distilled water
b) 2 Squirt bottles
c) Timer
WARNINGS TO THE USERS OF REAGENTS AND ANTIGEN COATED MICROPLATES
● Handle all reagents and samples as biohazardous material. It is recommended to dispose reagents and contaminated material according to the applicable regulations.
● Wear suitable protective clothing.
● Irritating to eyes and skin. Keep all reagents away from eyes and skin. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
● Take care not to contaminate any test reagents with samples or bacterial agents.
● The best results are achieved by following the protocols described below, using good, safe laboratory techniques.
● Never add water to the antigen coated microplates, conjugate, controls, or substrate.
● Do not use this kit or any of its contents after the expiration date.
● Do not intermix components from different serial numbers.
● Use a separate sample loop for each sample.
● Do not expose kit to direct sunlight.
● NEVER PIPETTE BY MOUTH. Harmful if swallowed.
● For animal use only.
NOTE: Allow all components to come to 21 - 25 °C before starting.
DANGER
Chromogenic Substrate: Danger. Causes serious eye irritation. May damage the unborn child. May cause cancer. Harmful to aquatic life with long lasting effects. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Wear protective gloves/protective clothing/eye protection/face protection. Wash thoroughly after handling. Avoid release to the environment. If exposed or concerned: Get medical advice/attention. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Store locked up. Dispose of contents/container in accordance with local/regional/national/international regulations.
SAMPLE COLLECTION AND STORAGE
● Follow proper sample collection procedures.
● Harvest serum and store properly (up to seven days at 2 - 7 °C).
● For prolonged storage, samples should be kept frozen (-20 °C or colder).
● Test only good quality serum or plasma. Severely hemolyzed or lipemic serum may produce background color. When in doubt, obtain a better quality sample.
PREPARATION OF WASH SOLUTION
Dilute wash concentrate 10-fold with distilled or deionized water (1 part concentrate to 9 parts deionized or distilled water) in a squirt bottle. Mix gently by inversion. Diluted wash solution may be stored at 2 - 7 °C.
TEST PROCEDURE
|
STEP |
NOTES |
|
|
SET UP AND SAMPLE INCUBATION |
||
|
1) |
Remove and place CDV wells (white rim) in top half of holder; one well for the Positive Control, one well for the Negative Control, and one well for each sample to be tested. |
 |
|
2) |
Place CPV wells (red rim) in bottom half of holder; one well for the Positive Control, one well for the Negative Control and one well for each sample to be tested. Leave the required number of wells attached to each other. |
|
|
3) |
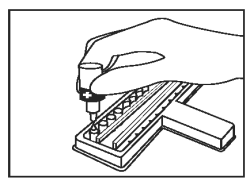
Add 1 drop of Positive Control (Red Cap) into the first CDV well and first CPV well. |
 |
|
4) |
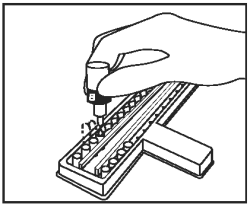
Add 1 drop of Negative Control/Sample Diluent (Bottle B - Gray Cap) to the second CDV well and to each CDV test sample well. Add 1 drop of Negative Control/Sample Diluent to the second CPV well and to each CPV test sample well. |
 |
|
5) |
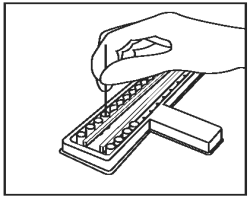
Using a separate sample loop for each sample, add 1 loopful (1 µL) of each sample to each of the CDV and CPV sample wells. Mix sample in diluent thoroughly by twisting the handle of the loop between the thumb and forefinger. Be careful not to splash from well to well. |
 |
|
6) |
Incubate for 5 minutes at 21 - 25 °C. |
|
|
7) |
NOTE: If several samples are run simultaneously, only one set of CDV and CPV controls is needed. |
|
|
BLOT AND WASH PROCEDURE |
||
|
8) |
Discard the fluid from all wells into an appropriate waste container. Wash wells once by vigorously filling the wells to overflowing with diluted wash solution. |
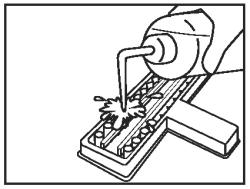 |
|
9) |
Discard excess fluid into an appropriate waste container. Invert holder and blot firmly onto a paper towel to remove remaining liquid. |
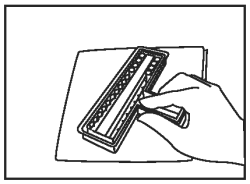 |
|
ADDITION OF CONJUGATE |
||
|
10) |
Add 1 drop of Conjugate (Bottle C - Blue Cap) into each well. Gently tap the holder for 10 - 15 seconds. |
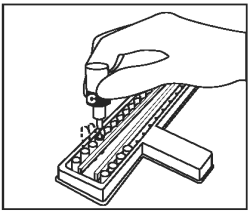 |
|
11) |
Incubate for 5 minutes at 21 - 25 °C. |
|
|
BLOT AND WASH PROCEDURE |
||
|
12) |
Discard the fluid from all wells into an appropriate waste container. Wash by vigorously filling the wells to overflowing with diluted wash solution. |
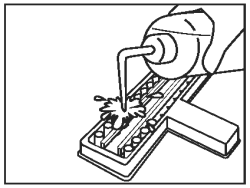 |
|
13) |
Discard the fluid from the wells into an appropriate waste container and blot after each wash. |
|
|
14) |
Repeat the wash procedure (steps 12 and 13) three (3) times. |
|
|
15) |
Wash two more times by vigorously filling the wells to overflowing with distilled or deionized water to remove bubbles. |
|
|
16) |
Discard excess fluid into an appropriate waste container. Invert holder and blot firmly onto a paper towel to remove residual liquid. |
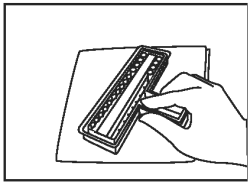 |
|
17) |
Washing is the most important step. Wells cannot be overwashed. Underwashing will result in color development in the negative control and negative sample wells. |
|
|
DEVELOP |
||
|
18) |
Place 2 drops of Chromogenic Substrate (Bottle D - Green Cap) into each well. Mix by gently tapping the holder several times. |
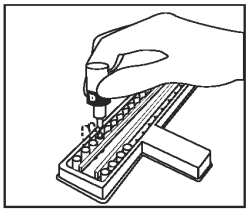 |
|
19) |
Incubate 5 minutes. |
|
|
20) |
After incubating, gently tap holder for 5 seconds and read results immediately. See Interpretation of Results section. |
|
|
|
NOTE: Prolonged incubation for more than 5 minutes in step 19 may result in nonspecific color development. If no color is seen after 5 minutes, the sample is negative. |
|
INTERPRETATION OF RESULTS
Compare each CDV sample well with the CDV positive and negative control wells. Compare each CPV sample well with the CPV positive and negative control wells.
Development of a blue color in the sample well that is of equal or greater intensity than the color of the Positive Control well is considered to be positive (CDV SN titer ≥ 1:16 or CPV HI titer ≥ 1:80).
No blue color in the sample well or color that is of less intensity than the color of the Positive Control Well is considered to be negative (CDV SN titer < 1:16 or CPV HI titer < 1:80).
For the test to be valid, the fluid in the positive control well must be distinctly blue, while that in the negative control well must show no color change from initial substrate color.
Results should be interpreted immediately after the 5 minute incubation period in Step 19.
Wells can be detached and compared alongside the positive control well using a white background for easier visual inspection.
NOTES
● Washing is a critical step. Wells cannot be overwashed. Underwashing will result in color development in the negative control and negative sample wells.
● Serum or plasma must be used as a sample.
● Hemolyzed and lipemic serum samples may be used; however, severely hemolyzed and lipemic samples may produce background color. When in doubt, obtain a better quality sample.
● Always compare results to the positive control. The kit negative control is used to verify good washing technique. It should not be used to differentiate positive from negative results.
SYMBOL DESCRIPTIONS

Zoetis Inc., Kalamazoo, MI 49007, USA
1-888-963-8471
www.zoetis.com
VLN/PCN 190/53P1.00
EC REP
ZOETIS FRANCE, 23 Rue Pierre Gilles de Gennes, 69007 Lyon, FRANCE
40016570
CPN: 3690616.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
