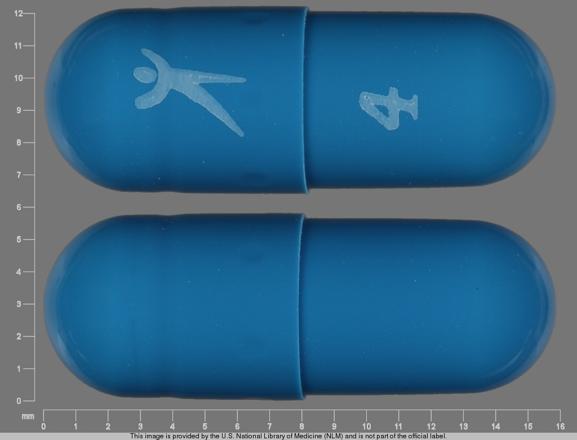
Logo 4 Pill - blue capsule/oblong, 16mm
Pill with imprint Logo 4 is Blue, Capsule/Oblong and has been identified as Tolterodine Tartrate Extended Release 4 mg. It is supplied by Greenstone LLC.
Tolterodine is used in the treatment of Urinary Incontinence; Overactive Bladder; Urinary Frequency and belongs to the drug class urinary antispasmodics. Risk cannot be ruled out during pregnancy. Tolterodine 4 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for Logo 4
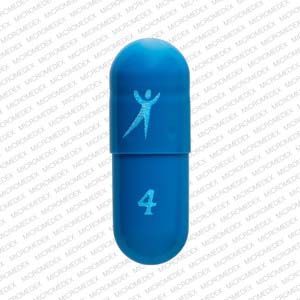
Tolterodine Tartrate Extended Release
- Imprint
- Logo 4
- Strength
- 4 mg
- Color
- Blue
- Size
- 16.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Urinary antispasmodics
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Greenstone LLC
- Inactive Ingredients
-
sucrose,
medium-chain triglycerides,
oleic acid,
FD&C Blue No. 2
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 59762-0048 | Viatris |
| 00009-5191 | Pfizer Inc. |
| 54868-4514 (Discontinued) | Physicians Total Care Inc. (repackager) |
| 54569-5205 | A-S Medication Solutions, LLC (repackager) |
| 55289-0132 | PDRX Pharmaceuticals Inc. (repackager) |
| 49999-0910 | Lake Erie Medical and Surgical Supply (repackager) |
| 35356-0417 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
Related images for "Logo 4"
More about tolterodine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (75)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: urinary antispasmodics
- Breastfeeding
- En español
Patient resources
- Tolterodine drug information
- Tolterodine (Advanced Reading)
- Tolterodine Tablets
- Tolterodine Extended-Release Capsules
Other brands
Professional resources
- Tolterodine monograph
- Tolterodine Tartrate Capsules (FDA)
- Tolterodine Tartrate ER Tablets (FDA)
- Tolterodine Tartrate Tablets (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.