Polyprenyl Immunostimulant
This treatment applies to the following species:This product license is conditional; efficacy and potency have not been fully demonstrated. For more information regarding safety data, see productdata.aphis.usda.gov. The package insert also contains additional information developed by the licensee.
U.S. Vet. Prod. No. 9380.00
U.S. Veterinary License No. 637
Polyprenyl Immunostimulant Indications
For the treatment of cats 8 weeks of age or older against rhinotracheitis. The product license is conditional; efficacy and potency have not been fully demonstrated. For more information regarding safety data, see productdata.aphis.usda.gov.
Description
Polyprenyl Immunostimulant is a 2 mg/ml solution of disodium salt of a homologous series of phosphorylated polyisoprenols with three trans- and an increasing number (7-9) of cis-isoprene isomers; its structure is shown here:
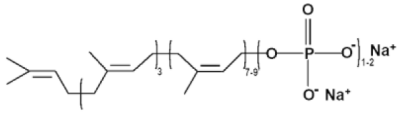
where 3 = number of trans-, 7-9 = number of cis- isomers;
1-2 = number of phosphoric anions
It is a colorless, clear liquid
DOSAGE AND ADMINISTRATION
The dose is 0.5 mg/kg (0.25 ml/kg) administered orally, twice daily for 15 days. Appropriate concurrent antibiotic use may be needed in the presence of secondary bacterial infections. Restricted to use by a veterinarian.
FELINE VIRAL RHINOTRACHEITIS
Feline viral rhinotracheitis (FVR) is a common upper respiratory disease in cats, caused by herpesvirus. Because FVR is very contagious, cats in multi-cat environments are at high risk of exposure to the virus. Cats of all ages may get infected. Chronic nasal and ocular problems often occur after herpesvirus infection.
The common signs of FVR are sneezing, loss of appetite, runny nose, watery eyes, conjunctivitis (inflammation of the tissues surrounding the eye, especially the lining of the lids and the third eyelid) and eye discharge, excessive salivation, mouth breathing, and lethargy.1
EFFICACY IN RHINOTRACHEITIS
Thirty-eight 6- to 11-week old, specific pathogen-free cats of both sexes were enrolled into a placebo-controlled, randomized, double-blinded virus challenge study. The cats were challenged with feline rhinotracheitis virus. Twenty randomly selected animals (experimental group) received 0.25 ml/kg (0.5mg/kg) of Polyprenyl Immunostimulant orally twice daily on days 0 through 14 days post-challenge (DPC); the other eighteen cats (placebo group) received an equal amount of placebo solution. The cats were examined for clinical signs daily, and clinical scores (0 = Absence, 1 = Moderate, clear, serous, 2 = Severe, mucopurulent) were recorded daily.
An animal was considered affected by FVR if a score of ≥1 for clinical signs of either rhinitis or conjunctivitis was present for one or more days.
|
Treatment |
Number of animals with |
|
|
Maximum Score=1 |
Maximum Score=2 |
|
|
Polyprenyl Immunostimulant |
11 |
9 |
|
Placebo |
3 |
15 |
SAFETY2
Safety studies in 390 owned cats total were conducted at 24 sites in 10 states comprising 3 geographical regions. Cats’ ages ranged from 2 days to 16 years including 128 cats ≤ 8 weeks of age. 0.5 mg/kg of Polyprenyl Immunostimulant was administered to the cats orally twice daily for 15 days. Adverse events were minimal, being confined to 3 animals reacting to the taste of the product and one animal having diarrhea.
The product has not been tested in pregnant animals. Do not mix with other products. Preservative: Amphotericin B.
SHIPPING AND STORAGE
Polyprenyl Immunostimulant is shipped in individual vials of 10 ml or in packages of 6 vials. Store at 2-8 °C (36 - 46 °F). Must not be frozen: freezing and thawing may cause precipitation of the product from the solution.
1Gaskell RM, Dawson S, Radford A. Chapter 16. Feline respiratory disease, In: Sykes J and Greene CE Infectious Diseases of The Dog And Cat, 5th Ed., 2022, Elsevier, St. Louis, MO.
2Legendre AM et al (2017) Frontiers Vet. Sci. doi: 10.3389/fvets.2017.00024, https://www.frontiersin.org/article/10.3389/fvets.2017.00024
Manufactured by: Sass & Sass, Inc., 115A Flint Rd., Oak Ridge, TN 37830
865-481-6000
U.S. Veterinary License # 637
Distributed by: VetImmune, LLC, P.O. Box 205, Kingston, TN 37763
The trademarks depicted herein are owned by their respective companies.
Copyright © 2020 Sass & Sass, Inc. All rights reserved.
6x10 ml vial (2 mg/ml)
CPN: 1819000.5
VetImmune, LLC, Panda Plus s.r.o.
115A FLINT RD., OAK RIDGE, TN, 37830
| Telephone: | 865-481-6000 | |
| Fax: | 865-940-0042 | |
| Website: | www.vetimmune.com | |
| Email: | info@sassandsass.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-08-26
