The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Novobrace
This page contains information on Novobrace for veterinary use.The information provided typically includes the following:
- Novobrace Indications
- Warnings and cautions for Novobrace
- Direction and dosage information for Novobrace
Novobrace
This treatment applies to the following species: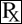 Company: Centaur
Company: Centaur
A SOFT TISSUE INJECTABLE BIOADHESIVE
Novobrace device for tendon and ligament injury stabilization in equines
Caution: Federal law restricts this device to sale by, or on the order of, a veterinarian
DEVICE DESCRIPTION
The Novobrace device consists of two vials, one containing a non-toxic protein crosslinking agent and the other a proprietary buffered carrier solution. Once reconstituted and injected the device assembles by polymerization and crosslinks damaged collagen fibers within and lining lesions. The device serves as an internal brace, supporting the injured area and preventing tear propagation while the tissue heals. By providing a protected environment for healing and by its ability to reduce incidences of re-injury in the compromised tissue, the Novobrace device can reduce return-to-work times by up to 50%.
INDICATIONS FOR USE
Treatment of tendonitis/desmitis or lesions in equine flexor tendons and suspensory ligaments.
CONTRAINDICATIONS
For equine veterinary use only. Not for use in humans. Not recommended for older horses with lesions above carpus or possible degenerative soft tissue disease.
Do not inject into synovial structures or lesions of greater than 70% surface area. Treat 48-72 hours after injury, coinciding with reduction of swelling.
CLINICAL PRECAUTIONS
Take care not to overfill lesion as leakage into the peri-tendonous region may result in crosslinking and inflammation.
It should be expected that using Novobrace will increase the size of repaired connective tissue to some degree, due to crosslinking in the tissue that Novobrace creates. Using no more than the recommended amount of Novobrace, and using sonography-guided injections to get the Novobrace directly to the site of connective tissue injury are the best ways to minimize this effect.
Rare but more severe inflammatory reactions may occur, the most common being a cellulitis within 6-12 hours. If this occurs, the attending veterinarian should re-examine the horse and assess. Treatment, at the discretion of the attending veterinarian, may involve systemic anti-inflammatory drugs, antibiotics, physical therapy and bandages. Such reactions commonly resolve within 24-48 hours.
For gray horses, horses with a history of sensitivity to injection, horses that are hyperactive or show/race horses where cosmetic appearance may be a particular concern, a regional limb perfusion (RLP) at the level of the carpus for foreleg and hock for hind limb, is recommended after administration of Novobrace. Use 4 mg of dexamethasone and 250 mg amikacin. The RLP should be performed over a 20 minute time period.
SAFETY
The crosslinking reaction produces a blue pigment that stains skin within one hour. Prompt washing of exposed skin after contact usually prevents staining. Use of gloves is therefore recommended whenever handling the product. Staining is harmless and fades within one week.
Avoid contact with eyes. Use of safety glasses during administration is strongly recommended. In case of contact with eyes, wash with copious amounts of water and seek medical advice.
HOW SUPPLIED
The Novobrace device is supplied in two sterile vials. Reconstitute immediately prior to use. The device is intended for single use only and degrades and rapidly loses efficacy upon reconstitution. Discard any unused solution. Store at or below 30°C.
Scan QR tag for educational video “Novobrace Technology: How It Works”
DIRECTIONS FOR USE
1. Inject horse with a weight appropriate dose of sedative.
2. Place a regional anesthesia block in the limb in order to desensitize the injection area.
3. Aseptically prepare injection site.
4. Using a sterile syringe and needle, remove 2.0 ml of carrier solution in Vial 2 and inject into Vial 1, allowing syringe pressure to equalize. Vial 2 must be at least room temperature (>65°F) prior to mixing.
5. Shake vigorously to dissolve the Novobrace Reagent.
● Note: Once reconstituted, the Novobrace Reagent is unstable. Use within 1 hour.
6. Using ultrasound guidance, place a 25g, 1 1/2 inch needle as described below. The preferred needle size is 25g. Do not use needles larger than 22g.
7. Attach syringe containing reconstituted solution to needle and inject slowly.
● Inject 0.2-0.3 ml into lesion and the same amount 3-4 cm proximal and 3-4 cm distal to the injury.
● For tendonitis, place 0.3 ml at one border of affected and normal regions, and inject 0.3 ml every 3 cm along the affected region. Place the last injection no more than 1 cm from the other border. Total volume should not exceed 1 ml.
● For suspensory ligaments, begin with one 0.2 ml injection in the center of the lesion/disrupted region. If closer than 2 cm to the sesamoid bone, then place no closer than 2 cm from the bone. This should cover 1.5 cm on either side of the injection site. If insufficient for complete coverage of affected area, place further 0.2 ml injections every 3 cm along ligament until final injection is not more than 1 cm from edge of affected area.
8. Apply a sterile bandage over the injection site and limb and replace daily for three days. A standing or stable bandage (non-sterile) may be used subsequently following this initial period.
9. At the discretion of the veterinarian, a weight appropriate dose of NSAIDs may be given to the horse for 3-5 days.
CROSSLINKING AGENT: GENIPIN
www.centauranimalhealth.com
For field support call 800-236-6180
CPN: 1488035.0
1351 - F WEST HWY 56, OLATHE, KS, 66061
| Telephone: | 913-390-6184 | |
| Order Desk: | 800-236-6180 | |
| Fax: | 913-390-5907 | |
| Website: | www.centauranimalhealth.com | |
| Email: | sales@centauranimalhealth.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
