The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
HESKA Feline UltraNasal FVRCP Vaccine
This page contains information on HESKA Feline UltraNasal FVRCP Vaccine for veterinary use.The information provided typically includes the following:
- HESKA Feline UltraNasal FVRCP Vaccine Indications
- Warnings and cautions for HESKA Feline UltraNasal FVRCP Vaccine
- Direction and dosage information for HESKA Feline UltraNasal FVRCP Vaccine
HESKA Feline UltraNasal FVRCP Vaccine
This treatment applies to the following species:Feline Rhinotracheitis-Calici-Panleukopenia Vaccine
Modified Live Virus, Code 16D1.22
For Use in Cats and Kittens Only
Description and General Information: Feline Rhinotracheitis-Calici-Panleukopenia Vaccine, MLV is designed for nasal administration. Each of the viruses are widespread and are common disease causing agents of cats.
HESKA Feline UltraNasal FVRCP Vaccine Indications
Recommended for the vaccination of healthy, susceptible cats against feline herpesvirus-1 (the cause of feline rhinotracheitis), feline calicivirus and feline parvovirus (the cause of feline panleukopenia). Cats can be vaccinated with a single dose at 12 weeks of age. If cats are vaccinated at less than 12 weeks of age, a second vaccination should be administered at 12 - 16 weeks of age. Annual revaccination is recommended.
Precautions
Store out of direct sunlight at a temperature between 35°F and 45°F (2°C - 7°C). Use the entire contents of the vaccine when first opened. Do not vaccinate pregnant animals. Do not use in kittens younger than 4 weeks of age. Burn containers and all unused contents.In rare instances, reactions can occur due to unusual sensitivity following use of this product. In such cases, administer epinephrine as an antidote. The vaccine will not protect against disease in the face of incubating feline herpesvirus, feline calicivirus or feline parvovirus infection. Therefore, only healthy cats should be considered as candidates for vaccination.
Transient sneezing for one or more days after vaccination is not uncommon. A small number of cats may experience other mild post-vaccination side effects including ocular and/or nasal discharge, GI signs (such as vomiting) or, in rare instances, nasal or oral ulcers. A transient slight drop in the white blood cell count has been noted at times after vaccination. These signs are usually mild and clear without treatment.
Contains gentamicin and fungistat as preservatives.
Dosage and Administration
Administer a single 0.2 ml dose intranasally, divided into both nostrils. NOTE: Use a new dropper for each cat vaccinated. No needles are necessary to administer the vaccine. This vaccine is NOT intended for intramuscular or subcutaneous injection.STEP 1: Remove the cap and rubber stopper from one vial of liquid vaccine and one vial of lyophilized vaccine.
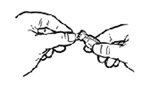
STEP 2: Using the nasal dropper, transfer the liquid vaccine (0.2 ml) to the lyophilized vaccine vial.
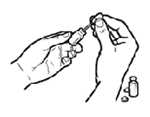
STEP 3: Mix the vaccine in the vial with a gentle swirling motion.
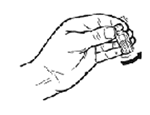
STEP 4: Once dissolved, immediately withdraw the rehydrated vaccine into the nasal dropper.
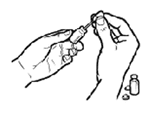
STEP 5: Vaccinate the cat by placing the vaccine equally in each nostril. Avoid touching the nose with the dropper.
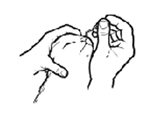
Contains 20 (1 dose) vials of dry vaccine, 20 (0.2 ml) vials of liquid vaccine and 20 droppers.
Keep refrigerated at a temperature between 35°F and 45°F. Store out of direct sunlight.
Heska Corporation
1-800-GO HESKA
Fort Collins, Colorado 80525
U.S. Vet. License No. 213
©2004 Heska Corporation
04178
904101
NAC No.: 14820111
3760 ROCKY MOUNTAIN AVENUE, LOVELAND, CO, 80538
| Telephone: | 970-493-7272 | |
| Fax: | 970-619-3008 | |
| Information/Order Desk: | 800-GO-HESKA | |
| Website: | www.heska.com | |
| Email: | market@heska.com |
 |
Every effort has been made to ensure the accuracy of the HESKA Feline UltraNasal FVRCP Vaccine information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |
Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
