Zelsuvmi: Package Insert / Prescribing Info
Package insert / product label
Generic name: berdazimer
Dosage form: topical gel
Drug class: Miscellaneous topical agents
Medically reviewed by Drugs.com. Last updated on Jul 13, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ZELSUVMI™ (berdazimer) topical gel
Initial U.S. Approval: 2024
Indications and Usage for Zelsuvmi
ZELSUVMI™ is a nitric oxide (NO) releasing agent indicated for the topical treatment of molluscum contagiosum (MC) in adults and pediatric patients 1 year of age and older. (1)
Zelsuvmi Dosage and Administration
Dosage Forms and Strengths
Topical gel: 10.3% berdazimer supplied as two tubes. Tube A contains berdazimer gel and Tube B contains hydrogel. (3)
Contraindications
None. (4)
Warnings and Precautions
Application Site Reactions: Application site reactions, including allergic contact dermatitis, occurred. Discontinue ZELSUVMI and initiate appropriate therapy. (5.1)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions (≥1%) are application site reactions, including pain (such as burning or stinging sensations, 18.7%), erythema (11.7%), pruritus (5.7%), exfoliation (5.0%), dermatitis (4.9%), swelling (3.5%), erosion (1.6%), discoloration (1.5%), vesicles (1.5%), irritation (1.2%), and infection (1.1%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact LNHC, Inc. at 1-855-330-7546 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2024
Full Prescribing Information
1. Indications and Usage for Zelsuvmi
ZELSUVMI™ is indicated for the topical treatment of molluscum contagiosum (MC) in adults and pediatric patients 1 year of age and older.
2. Zelsuvmi Dosage and Administration
2.1 Important Preparation and Administration Instructions
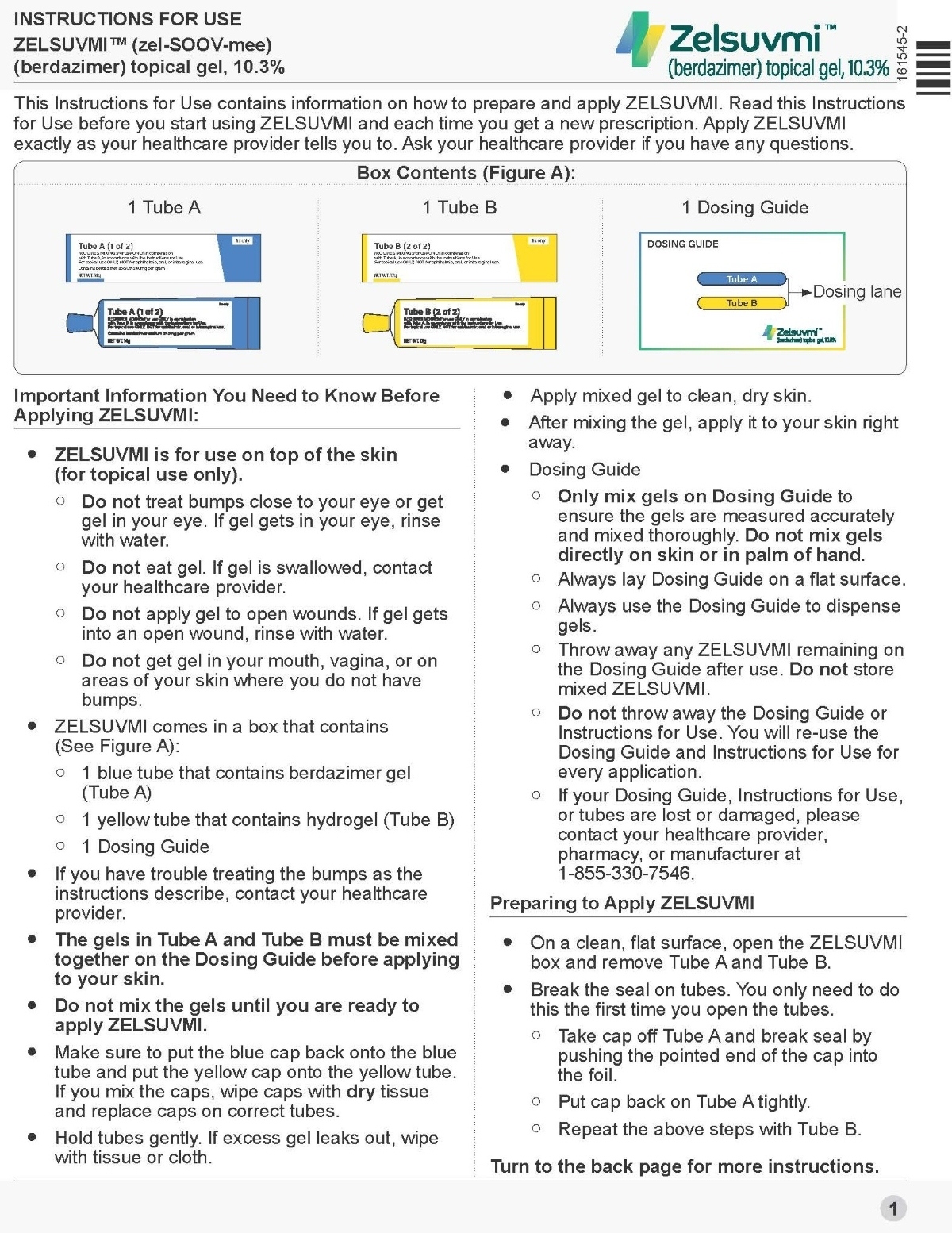
- ZELSUVMI is supplied in a carton containing the following:
- Tube A containing berdazimer gel
- Tube B containing hydrogel
- Dosing guide
- Mix together equal amounts of gel from Tube A and Tube B before application [see Dosage and Administration (2.2)].
- Do not premix or store mixed ZELSUVMI.
- Instruct the patient to refer to the ZELSUVMI “Instructions for Use” for detailed instructions on the preparation and administration of ZELSUVMI [see Instructions for Use].
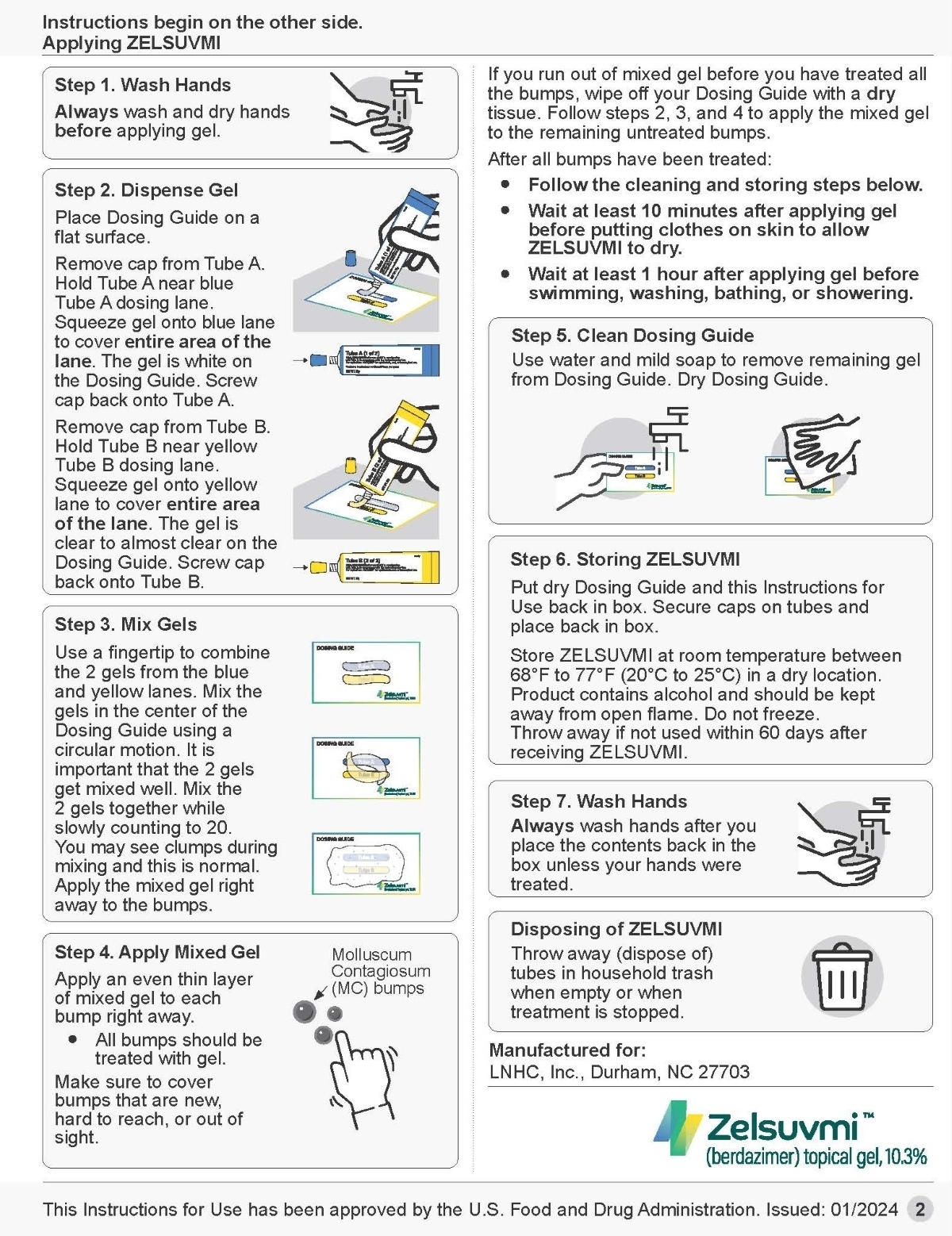
2.2 Recommended Dosage and Administration
- Dispense equal amounts (0.5 mL) of gel from Tube A and Tube B on the dosing guide. Immediately put the caps back on Tube A and Tube B tightly.
- Mix together on the dosing guide.
- Immediately apply ZELSUVMI as an even thin layer. Apply ZELSUVMI once daily to each MC lesion for up to 12 weeks.
- Wash hands after applying ZELSUVMI, unless hands are being treated.
- Allow ZELSUVMI to dry for 10 minutes after application.
- Avoid application to uninvolved skin and avoid transfer of applied ZELSUVMI to other areas, including the eye.
- Avoid swimming, bathing, or washing for 1 hour after application of ZELSUVMI.
- ZELSUVMI is for topical use only and not for ophthalmic, oral, or intravaginal use.
3. Dosage Forms and Strengths
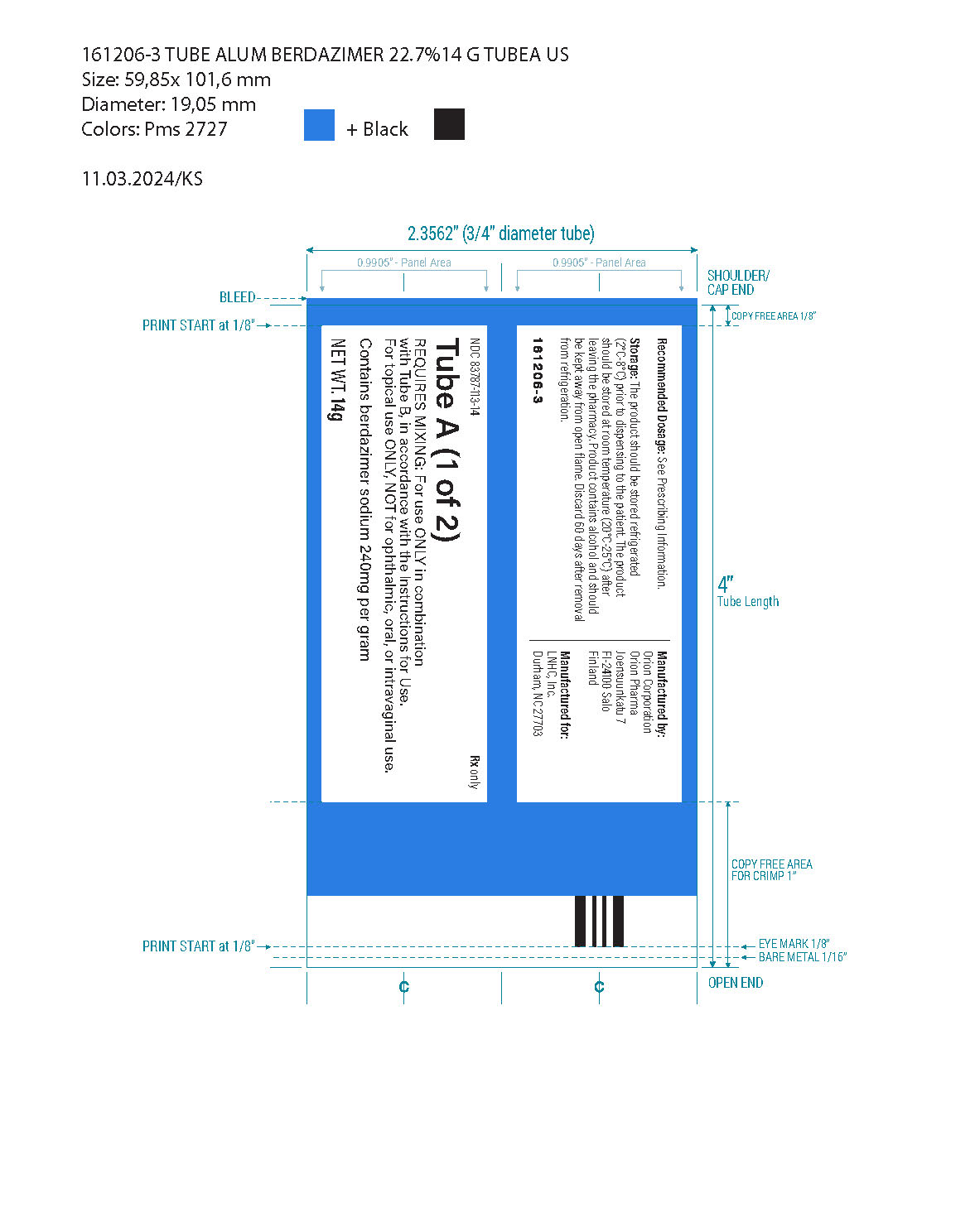
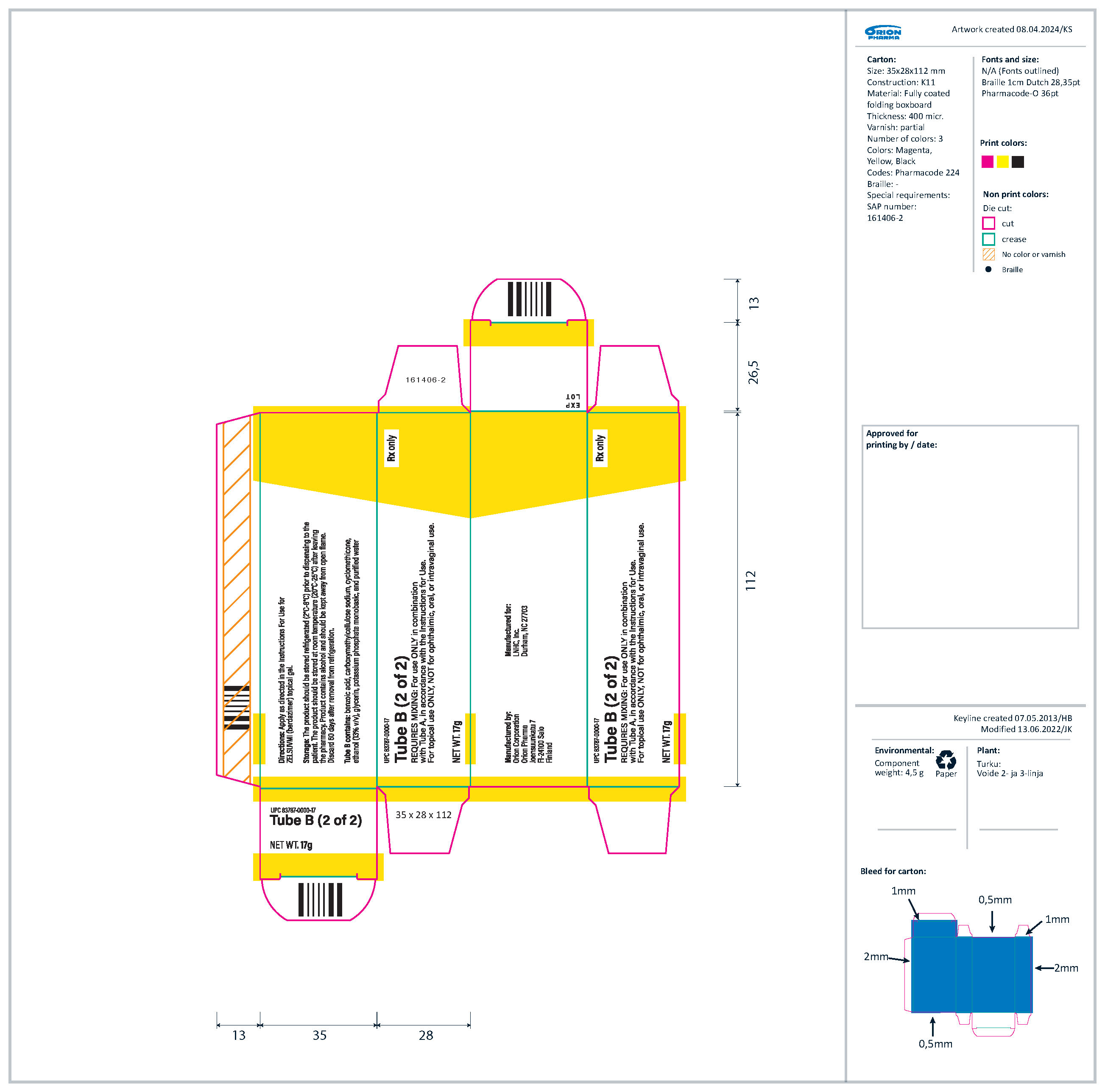
Topical gel: 10.3% berdazimer in an opaque white to off-white gel. ZELSUVMI is supplied as two tubes. Tube A with a blue label contains 14 grams of berdazimer gel and Tube B with a yellow label contains 17 grams of hydrogel.
5. Warnings and Precautions
5.1 Application Site Reactions
Application site reactions, including allergic contact dermatitis, have occurred in patients treated with ZELSUVMI. Suspect allergic contact dermatitis in the event of pain, pruritus, swelling or erythema at the application site lasting longer than 24 hours. If allergic contact dermatitis occurs, discontinue ZELSUVMI and initiate appropriate therapy.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In three double-blind, vehicle-controlled clinical trials (Trial 1, and Trial 2 and Trial 3, which were similarly designed to Trial 1), 1596 adult and pediatric subjects were treated with ZELSUVMI or vehicle gel topically once daily for up to 12 weeks [see Clinical Studies (14)]. In these trials 3% of subjects were less than 2 years of age, and 96% of subjects were 2 to 17 years of age. The trial population included 51% male, 88% White, 6% Black, and 6% Other; for ethnicity, 21% of subjects identified as Hispanic/Latino, 78% as non-Hispanic/Latino, and 1% were not reported. Adverse reactions reported by ≥1% of subjects and more frequently than vehicle-treated subjects are listed in Table 1.
| ZELSUVMI
N=916 |
Vehicle Gel N=680 |
|||||
| Adverse Reaction | Mild
n (%) | Moderate
n (%) | Severe
n (%) | Mild
n (%) |
Moderate n (%) |
Severe n (%) |
| Subjects with any TEAE* | 220 (24.0) | 192 (21.0) | 16 (1.7) | 118 (17.4) | 47 (6.9) | 4 (0.6) |
| Application Site Pain† | 113 (12.3) | 56 (6.1) | 2 (0.2) | 30 (4.4) | 3 (0.4) | 0 |
| Application Site Erythema | 48 (5.2) | 55 (6.0) | 4 (0.4) | 7 (1.0) | 2 (0.3) | 0 |
| Application Site Pruritus | 36 (3.9) | 15 (1.6) | 1 (0.1) | 5 (0.7) | 2 (0.3) | 0 |
| Application Site Exfoliation | 18 (2.0) | 26 (2.8) | 2 (0.2) | 0 | 0 | 0 |
| Application Site Dermatitis | 16 (1.7) | 26 (2.8) | 3 (0.3) | 3 (0.4) | 2 (0.3) | 0 |
| Application Site Swelling | 17 (1.9) | 14 (1.5) | 1 (0.1) | 3 (0.4) | 1 (0.1) | 0 |
| Pyrexia | 14 (1.5) | 6 (0.7) | 0 | 6 (0.9) | 1 (0.1) | 0 |
| Application Site Erosion | 7 (0.8) | 5 (0.5) | 3 (0.3) | 1 (0.1) | 0 | 0 |
| Application Site Discoloration | 13 (1.4) | 1 (0.1) | 0 | 1 (0.1) | 0 | 0 |
| Application Site Vesicles | 5 (0.5) | 9 (1.0) | 0 | 0 | 1 (0.1) | 0 |
| Vomiting | 5 (0.5) | 7 (0.8) | 0 | 1 (0.1) | 0 | 0 |
| Application Site Irritation | 7 (0.8) | 4 (0.4) | 0 | 0 | 0 | 0 |
| Upper Respiratory Tract Infection | 6 (0.7) | 5 (0.5) | 0 | 4 (0.7) | 1 (0.1) | 0 |
| Application Site Infection | 4 (0.4) | 4 (0.4) | 2 (0.2) | 2 (0.3) | 1 (0.1) | 0 |
| * TEAE – treatment emergent adverse events † Application site pain also includes application site burning and stinging. |
||||||
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on ZELSUVMI use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of berdazimer to pregnant rats and rabbits increased malformations in the presence of severe maternal toxicity (see Data). The clinical relevance of this finding is unknown given the bioavailability of berdazimer following oral administration is significantly higher than topical application.
The available data do not allow the calculation of relevant comparisons between the systemic exposure of berdazimer observed in animal studies and the systemic exposure that would be expected in humans after topical use of ZELSUVMI.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Systemic embryo-fetal development studies were conducted in rats and rabbits. In an embryo-fetal development study in rats, oral dose levels of 28, 95, or 189 mg/kg/day berdazimer were administered during the period of organogenesis. Maternal mortality and elevated methemoglobin levels were noted in dams receiving doses of 95 and 189 mg/kg/day. The maternal no observable adverse effect level (NOAEL) was 28 mg/kg/day. Fetal skeletal malformations (changes in the lumbar and thoracic centra or arches, missing thoracic arches and centra, additional bone in the thoracic arches, missing lumbar centra and arches, and fused ribs) and visceral malformations (cleft palate) and decreased fetal weights were observed in litters from dams receiving 189 mg/kg/day. The fetal NOAEL was 95 mg/kg/day.
In an embryo-fetal development study in rabbits, oral dose levels of 47, 142, or 284 mg/kg/day berdazimer were administered during the period of organogenesis. Maternal mortality, aborted fetuses, adverse clinical observations, and elevated methemoglobin levels were noted in pregnant rabbits receiving doses of 142 and 284 mg/kg/day. The maternal NOAEL was 47 mg/kg/day. Decreased fetal weights were noted from pregnant rabbits receiving 284 mg/kg/day. The fetal NOAEL was 142 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of berdazimer or its metabolite in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZELSUVMI and any potential adverse effects on the breastfed infant from ZELSUVMI or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ZELSUVMI for the topical treatment of MC have been established in pediatric patients 1 year of age and older. Use of ZELSUVMI for this indication is supported by data from three randomized, vehicle-controlled, double-blind trials involving 1596 subjects of which 1575 were pediatric subjects with MC (904 were exposed to ZELSUVMI; 29 subjects were less than 2 years of age, including one subject less than 1 year of age, and 875 were 2 to 17 years of age) [see Clinical Studies (14)].
The safety and effectiveness of ZELSUVMI have not been established in pediatric patients younger than 1 year of age.
8.5 Geriatric Use
Of the total number of ZELSUVMI-treated subjects in clinical studies for MC, none were 65 to 74 years of age, and one was 75 years of age and older [see Clinical Studies (14)]. Clinical studies of ZELSUVMI did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
11. Zelsuvmi Description
ZELSUVMI (berdazimer) topical gel, 10.3%, a nitric oxide releasing agent, contains the drug substance berdazimer sodium, a white to off white powder with the chemical name poly[{[3-(methylamino)propyl]silasesquioxane}-co-{[3-(1-methyl-2-nitroso2-oxidohydrazin-1 yl)propyl]silasesquioxane}-co-silicate (1:3:6 x)], partially hydrolyzed (Si : OH ~ 10 : 5), and the following structural and empirical formula:
Structural Formula:
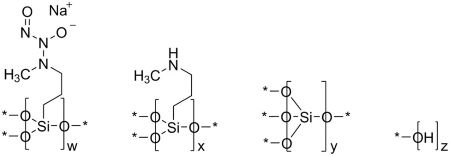
* Denotes shared oxygen atom between bonded constituents; resulting bonds are Si-O-Si or Si‑OH
Empirical formula: [(C4H9N3NaO3.5Si)3(C4H10NO1.5Si)1(SiO2)6(HO0.5)5]0.1n
Due to the insoluble nature of berdazimer sodium, the molecular formula, molecular mass, and average molecular weight range cannot be determined.
ZELSUVMI (berdazimer) topical gel is an opaque white to off-white gel containing 10.3% berdazimer (equivalent to 10.9% berdazimer sodium). ZELSUVMI is supplied as two gel components that are mixed before administration:
- Tube A (14 g): an opaque white to off-white gel containing 240 mg of berdazimer sodium per gram of gel and the inactive ingredients cyclomethicone, hexylene glycol, hydroxypropyl cellulose, and isopropyl alcohol.
- Tube B (17 g): a translucent to opaque white to off-white gel containing the inactive ingredients benzoic acid, carboxymethylcellulose sodium, cyclomethicone, ethanol (13% v/v), glycerin, potassium phosphate monobasic, and purified water.
12. Zelsuvmi - Clinical Pharmacology
12.1 Mechanism of Action
ZELSUVMI is a nitric oxide releasing agent. The mechanism of action for the treatment of molluscum contagiosum is unknown.
12.3 Pharmacokinetics
Plasma hydrolyzed MAP3 (hMAP3), a structural marker for berdazimer, and nitrate levels were evaluated in n=34 subjects 2 to 12 years of age with MC. Subjects applied ZELSUVMI once-daily for two weeks to a total treatment area of 484 cm2 (mean lesion count=34), applying a mean dose of approximately 3 mL/day. No subjects had quantifiable plasma hMAP3 concentrations on day 1; two subjects had quantifiable concentrations on day 15. Mean plasma nitrate levels were similar on days 1 and 15 and remained relatively flat during the PK sampling period (baseline through 1, 3, and 6 hours post-application).
There were no apparent differences in methemoglobin levels throughout the study.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of berdazimer gel was assessed in a 2-year dermal mouse carcinogenicity study. There were no drug-related tumor findings associated with daily topical administration of berdazimer gel to mice at doses up to 4% berdazimer gel.
Berdazimer was mutagenic in a bacterial mutagenicity assay (Ames assay) but was not clastogenic in an in vitro chromosomal aberration assay in human peripheral blood lymphocytes or in an in vivo micronucleus assay in rats.
There were no berdazimer related effects on male or female fertility and early embryonic parameters in rats at oral doses up to 189 mg/kg/day.
14. Clinical Studies
The efficacy of ZELSUVMI was evaluated in 3 multicenter, randomized, double-blind, parallel-group, vehicle-controlled trials in subjects with MC (Trials 1, 2, and 3; NCT04535531, NCT03927703, and NCT03927716, respectively). Trial 1 enrolled 891 subjects, Trial 2 enrolled 355 subjects, and Trial 3 enrolled 352 subjects. Subjects were randomized 1:1 in Trial 1, and 2:1 in Trials 2 and 3 to receive ZELSUVMI or vehicle applied to MC lesions once daily for up to 12 weeks.
In the three trials, 3% of subjects were less than 2 years of age and 96% of subjects were 2 to 17 years of age. The trial population included 51% male, 88% White, 6% Black, and 6% Other; for ethnicity, 21% of subjects identified as Hispanic/Latino, 78% as non-Hispanic/Latino, and 1% were not reported. Subjects had 3-70 baseline MC lesions. At baseline, the average MC lesion count was 20.2.
The primary efficacy endpoint was the proportion of subjects achieving complete clearance at Week 12. Complete clearance was defined as the subject having a total MC lesion count of 0 at assessment. The key secondary efficacy endpoint was complete clearance rate at Week 8.
Efficacy was demonstrated in Trials 1 and 2. The results are summarized in Table 2.
| Trial 1 | Trial 2 | |||
|
ZELSUVMI (N=444) |
Vehicle (N=447) |
ZELSUVMI (N=237) |
Vehicle (N=118) |
|
| Complete Clearance Rate at Week
12 (Primary Endpoint) | 32.4% | 19.7% | 30.0% | 20.3% |
|
Treatment Difference (95% Confidence Interval) |
12.8% (7.1%, 18.6%) |
9.2% (-0.04%, 18.4%) |
||
|
Complete Clearance Rate at Week 8 (Secondary Endpoint) | 19.6% | 11.6% | 13.9% | 5.9% |
|
Treatment Difference (95% Confidence Interval) |
7.5% (3.0%, 12.0%) |
7.8% (1.8%, 13.8%) |
||
In Trial 3, the complete clearance rates at Week 12 were 26% versus 22% for ZELSUVMI and vehicle, respectively, with 95% confidence interval (-5%, 14%).
16. How is Zelsuvmi supplied
How Supplied
ZELSUVMI (berdazimer) topical gel, 10.3% is supplied in a carton (NDC 83787-103-31) containing:
- Tube A (14 g) with blue label containing berdazimer sodium in an opaque white to off-white gel (NDC 83787-113-14)
- Tube B (17 g) with yellow label containing translucent to opaque white to off-white gel (UPC 83787-0000-17)
- Dosing Guide
Storage and Handling
- Prior to Dispensing: Store ZELSUVMI in a refrigerator between 2°C and 8°C (36°F and 46°F) until dispensed to the patient. Write the “Discard after” date in the space provided on the carton.
- After Dispensing: Store ZELSUVMI at room temperature, between 20°C to 25°C (68°F and 77°F) in a dry location.
- Product contains alcohol and should be kept away from open flame.
- Do not freeze.
- Discard 60 days after removal from refrigeration.
17. Patient Counseling Information
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Application Site Reactions
Advise patients to discontinue ZELSUVMI and seek medical attention immediately if signs or symptoms of application site reactions lasting more than 24 hours occur [see Warnings and Precautions (5.1)].
Administration Instructions
Advise patients that ZELSUVMI is for external use only and is not for ophthalmic, oral, or intravaginal use [see Dosage and Administration (2.2)].
Inform patients that ZELSUVMI is supplied with two gel components that must be mixed together immediately before application. Instruct patients not to premix or store mixed ZELSUVMI [see Dosage and Administration (2.1, 2.2)].
Advise patients to wash hands after applying ZELSUVMI (unless hands are being treated) and avoid transfer of the product to other areas of the skin, including the eye [see Dosage and Administration (2.2)].
Instruct patients to carefully follow the instructions for preparing and administering ZELSUVMI in the ZELSUVMI FDA-approved patient labeling [see Instructions For Use].
Manufactured for:
LNHC, Inc.
Durham, NC 27703
Patient Package Insert
| PATIENT INFORMATION
ZELSUVMI™ (zel-SOOV-mee) (berdazimer) topical gel |
|
| Important information: ZELSUVMI is for use on the skin (for topical use) only. Do not use ZELSUVMI near or in your eyes, mouth, or vagina. | |
|
What is ZELSUVMI? ZELSUVMI is a prescription medicine used on the skin (topical) to treat molluscum contagiosum (MC) in adults and children 1 year of age and older. It is not known if ZELSUVMI is safe and effective in children under 1 year of age. |
|
|
Before using ZELSUVMI, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
|
How should I use ZELSUVMI?
|
|
| What are the possible side effects of ZELSUVMI?
ZELSUVMI may cause serious side effects, including:
The most common side effects of ZELSUVMI include: |
|
|
|
|
These are not all of the possible side effects of ZELSUVMI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store ZELSUVMI?
|
|
|
General information about the safe and effective use of ZELSUVMI.
|
|
|
What are the ingredients in ZELSUVMI? Tube A Active ingredient: berdazimer sodium Inactive ingredients: cyclomethicone, hexylene glycol, hydroxypropyl cellulose, and isopropyl alcohol Tube B Inactive ingredients: benzoic acid, carboxymethylcellulose sodium, cyclomethicone, ethanol, glycerin, potassium phosphate monobasic, and purified water Manufactured for: LNHC, Inc. Durham, NC 27703 U.S. Patents: www.pelthos.com/patents For more information, call 1-855-330-7546 or go to www.Zelsuvmi.com. |
|
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 01/2024
| ZELSUVMI
berdazimer kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - LNHC, Inc. (119105589) |
More about Zelsuvmi (berdazimer topical)
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous topical agents
- Breastfeeding