TissueBlue: Package Insert / Prescribing Info
Package insert / product label
Generic name: brilliant blue g
Dosage form: injection, solution
Drug class: Ophthalmic diagnostic agents
Medically reviewed by Drugs.com. Last updated on Aug 25, 2025.
On This Page
Highlights of Prescribing Information
TISSUEBLUE (Brilliant Blue G Ophthalmic Solution) 0.025%, for intraocular ophthalmic use
Initial U.S. Approval: 2019
Indications and Usage for TissueBlue
TissueBlue 0.025% is a disclosing agent indicated to selectively stain the internal limiting membrane (ILM). (1)
TissueBlue Dosage and Administration
- Inject TissueBlue 0.025% directly in a Balanced Salt Solution (BSS)-filled vitreous cavity.
- Excess TissueBlue should be removed from the vitreous cavity.
Dosage Forms and Strengths
TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is supplied in 2.25 mL syringes filled to a volume of 0.5 mL. (3)
Contraindications
None (4)
Warnings and Precautions
Excessive staining: Excess TissueBlue 0.025% should be removed from the eye immediately after staining.
Use of the syringe: Make sure the plunger moves smoothly before injecting the solution.
(5)
Adverse Reactions/Side Effects
Adverse reactions that have been reported in procedures that included the use of TissueBlue 0.025% have often been associated with the surgical procedure. The complications include retinal (retinal break, tear, hemorrhage, and detachment and cataracts. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Dutch Ophthalmic, USA at 1-800-75-DUTCH or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch
(6)
Revised: 5/2025
Full Prescribing Information
Indications and Usage for TissueBlue
TissueBlue 0.025% is indicated to selectively stain the internal limiting membrane (ILM).
TissueBlue Dosage and Administration
TissueBlue 0.025% is carefully injected into the Balanced Salt Solution (BSS)-filled vitreous cavity using a blunt cannula attached to the pre-filled syringe, without allowing the cannula to contact the retina or allowing TissueBlue to get under the retina. Sufficient staining is expected within a few seconds. Following staining, all excess dye should be removed from the vitreous cavity.
Dosage Forms and Strengths
TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is a clear, bright blue, single-dose ophthalmic solution supplied in 2.25 mL syringes pre-filled to a volume of 0.5 mL.
Warnings and Precautions
Excessive Staining
Excess TissueBlue 0.025% should be removed from the eye immediately after staining.
Use of the Syringe
Make sure the plunger moves smoothly before injecting the solution. Do not use the product if the plunger does not move smoothly to prime the cannula.
Adverse Reactions/Side Effects
Adverse reactions that have been reported in procedures that included the use of Brilliant Blue G Ophthalmic Solution have often been associated with the surgical procedure. These complications include retinal (retinal break, tear, hemorrhage, and detachment) and cataracts.
Related/similar drugs
Use In Specific Populations
TissueBlue 0.025% - Pregnancy section
Risk Summary
There are no available data on the use of TissueBlue 0.025% in pregnant women to inform a drug associated risk. Systemic absorption of TissueBlue 0.025% in humans is expected to be negligible following intravitreal injection and subsequent removal of the drug at the completion of surgical procedures. Due to the negligible systemic exposure, it is not expected that maternal use of TissueBlue 0.025% will result in fetal exposure to the drug.
Adequate animal reproduction studies were not conducted with TissueBlue 0.025%.
TissueBlue 0.025% - Lactation section
Risk Summary
No data are available regarding the presence of Brilliant Blue G in human milk after intraocular administration of TissueBlue 0.025%, or the effects on the breastfed infant or the effects on milk production. However, breastfeeding is not expected to result in exposure of the child to Brilliant Blue G due to the expected negligible systemic exposure of BBG in humans following intravitreal injection and subsequent removal of the drug at the completion of surgical procedures.
TissueBlue Description
TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is a sterile solution of BBG (a dye) for intraocular ophthalmic use. Each mL of TissueBlue 0.025% contains BBG 0.25 mg, polyethylene glycol and buffered sodium chloride solution (sodium chloride, dibasic sodium phosphate, monobasic sodium phospage, water for injection). Phosphoric acid and/or sodium hydroxide may also be used for pH adjustment. The pH range of TissueBlue 0.025% Solution is between 7.3 and 7.6.
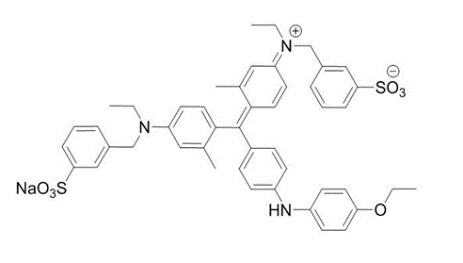
The drug substance BBG has the chemical name Brilliant Blue G, a molecular weight of 854.02 and has the following chemical structure:
Molecular formula: C 47H 48N 3NaO 7S 2
Nonclinical Toxicology
TissueBlue 0.025% - Nonclinical toxicology section
Studies to evaluate the potential for carcinogenicity or impairment of fertility of TissueBlue 0.025% have not been conducted.
Brilliant Blue G was not mutagenic in the Ames assay, the in vitro mouse lymphoma assay, or the in vivo rat micronucleus assay.
How is TissueBlue supplied
TissueBlue (Brilliant Blue G Ophthalmic Solution), 0.025% is supplied as 0.5 mL of Brilliant Blue G Ophthalmic Solution, 0.025% in a sterile, single-dose Luer Lok, 2.25 mL glass syringe, with a grey rubber plunger stopper and tip cap with polypropylene plunger rod in a pre-formed polypropylene blister pouch sealed with a Tyvek® lid.
NDC 68803-722-05 (One 0.5 mL syringe)
NDC 68803-722-25 (Carton of five 0.5 mL syringes)
Storage and Handling
TissueBlue 0.025% should be stored at 15°C to 25°C (59°F to 77°F). Protect from light, frost and moisture.
Distributed by:
Dutch Ophthalmic, USA
10 Continental Drive, Bldg 1
Exeter, NH 03833, USA
Phone: 800-75-DUTCH or 603-778-6929
Made in Germany
All trademarks are the property of their respective owners.
Package Label - 0.5 mL
TissueBlue
(Brilliant Blue G Ophthalmic Solution) 0.025%
Staining Solution for Ophthalmic Surgery
Protect from light, frost and moisture. Store at 15°C to 25°C (59°F to 77°F). Sterile.
Active ingredients: Brilliant Blue G 0.025% Inactive ingredients: Water for injection, Sodium chloride, dibasic sodium phosphate, monobasic sodium phosphate, polyethylene glycol, phosphoric acid and/or sodium hydroxide may also be used for pH adjustment.
NDC 68803-722-05 (One 0.5 mL syringe)
NDC 68803-722-25 (Carton of five 0.5 mL syringes)
| TISSUEBLUE
brilliant blue g injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - D.O.R.C. Dutch Ophthalmic Research Center (International) B.V. (407522184) |
| Registrant - D.O.R.C. Dutch Ophthalmic Research Center (International) B.V. (407522184) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmpur GmbH | 340805167 | manufacture(68803-722) | |
More about TissueBlue (brilliant blue g ophthalmic)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: ophthalmic diagnostic agents
