Home FDA PI Sterile water
Sterile Water for Inhalation: Package Insert / Prescribing Info
Package insert / product label Dosage form: inhalation solutionDrug class: Sterile irrigating solutions
Medically reviewed by Drugs.com. Last updated on Nov 28, 2024.
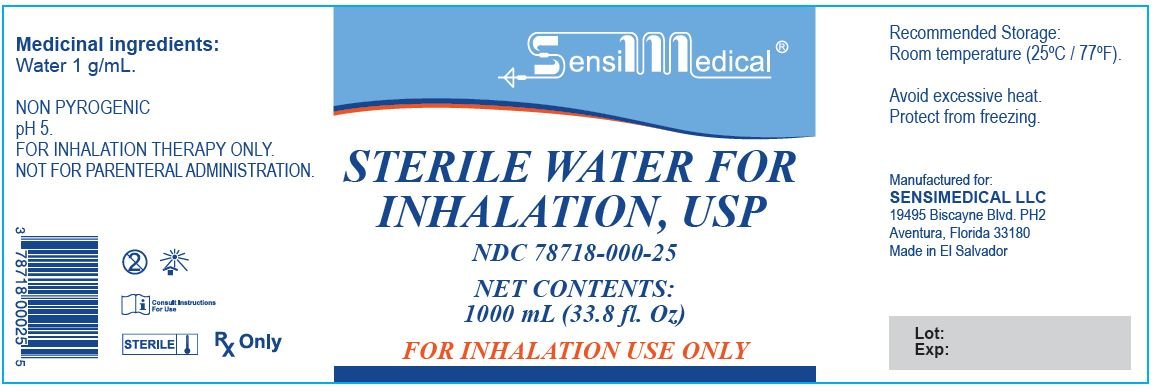
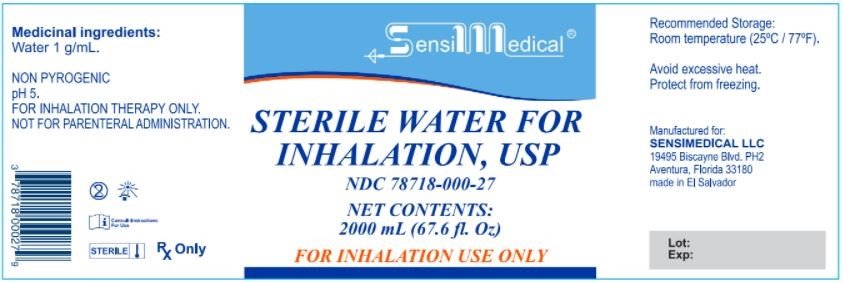
Medicinal Ingredient: Water 1 g/mL
NON PYROGENIC
pH 5
FOR INHALATION THERAPY ONLY
NOT FOR PARENTAREL ADMINISTRATION
Recommended Storage: Room temperature (25°C/77°F)
Avoid Excessive Heat. Protect from freezing.
Manufactured For:
SENSIMEDICAL LLC
19495 Biscayne Blvd PH2
Aventura, Florida 33180
Made in El Salvador
STERILE WATER FOR INHALATION
sterile water for inhalation inhalant
Related/similar drugs
Sterile water Biosimilars
Biosimilar and interchangeable products are biological products that are highly similar to and have no clinically meaningful differences from the reference product.
Reference products
These are biological products that have already been approved by the FDA, against which biosimilar products are compared. There are 3 for sterile water.
Jynneos (Smallpox and Mpox Vaccine, Live, Non-replicating) - Bavarian Nordic A/S
Formulation type
Strength
Single-Dose Vial
0.5-3.95 X 10 E8 INF. U/.5 mL
Single-Dose Vial
0.5-3.95 X 10 E8 INF. U/.5 mL
M-M-R Ii (Measles, Mumps and Rubella Virus Vaccine Live) - Merck Sharp & Dohme LLC
Formulation type
Strength
Single-Dose Vial
0.5 mL
Single-Dose Vial
0.5 mL
Zostavax (Zoster Vaccine Live) - Merck Sharp & Dohme LLC
Formulation type
Strength
Single-Dose Vial
0.65 mL Discontinued
Medical Disclaimer