ROTOP - DMSA: Package Insert / Prescribing Info
Package insert / product label
Generic name: kit for the preparation of technetium tc99m succimer injection
Dosage form: injection, powder, lyophilized, for solution
THERAGNOSTICS
Theragnostics Inc.
150 Grossman Drive, Suite 306
Braintree, MA 02184
IMPORTANT ORDERING INFORMATION
December 1, 2020
Subject: New Ordering Procedures for ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection
Dear Healthcare Professional,
Please note the following information prior to placing an order for ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection. On September 1, 2020, GE Healthcare sold their radiopharmacy chain to a third party, Radioisotope Life Sciences (RLS), and no longer have the capability to make radiolabelled 99mTc-DMSA.
To place an order for ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (Theragnostics), please contact your local nuclear pharmacy, ex. Cardinal Health, Jubilant, RLS, UPPI, Pharmalogic or another independent nuclear pharmacy. You can also contact Medi-Physics Inc., dba GE Healthcare, 1-800-292-8514, 8:00 AM to 6:30 PM Eastern time; or via web portal address ci.gehealthcare.com/us.
CONTACT NUMBERS: Please use the following contact numbers as appropriate:
Phone: 1-617-286-7479
Fax: 1-617-398-6337
Sincerely,
Gregory Mullen
President & CEO
THERAGNOSTICS
Theragnostics Inc.
150 Grossman Drive, Suite 306
Braintree, MA 02184
IMPORTANT PRESCRIBING INFORMATION
December 1, 2020
Subject: Temporary importation of Kit for the Preparation of Technetium Tc99m Succimer Injection to address drug shortage issues
Dear Healthcare Professional,
Due to the current critical shortage of DMSA Kit for the Preparation of Technetium Tc99m Succimer, Theragnostics Inc. (Theragnostics) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. Theragnostics has initiated temporary importation of DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection into the U.S. market. This product is marketed in Germany and is manufactured in Dresden, Germany by ROTOP Pharmaka GmbH for Theragnostics.
At this time, no other entity except ROTOP Pharmaka GmbH, Germany through its US Agent, Theragnostics, and Theragnostics' distributor, Medi-Physics Inc., dba GE Healthcare, is authorized by the FDA to import or distribute the DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection in the U.S. FDA has not approved ROTOP Pharmaka GmbH's Kit for DMSA Preparation of Technetium Tc99m Succimer Injection product in the U.S.
Effective immediately, and during this temporary period, Theragnostics will offer the following presentation of ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection:
| Product | Strength | Size | Marketing Authorization # |
|---|---|---|---|
| ROTOP DMSA (Kit for the Preparation of Technetium Tc99m Succimer Injection) | One vial contains 1.74 mg powder with the active substance: 1.0 mg succimer | 5 vials in a carton | 3003663.00.00 Germany (NDC 71647-001-01) |
The vial and carton labels will display the text, translated to English, as approved via the Marketing Authorization of EEA in Germany. At the end of this letter you will find a product comparison table with the prescribing information in English, as well as images of the labels for your reference.
There are some differences in the labeling between the FDA-approved DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (GE Healthcare) product and ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (Theragnostics) product (please see the product comparison tables below). These differences do not alter the favorable risk/benefit of the drug:
- In alignment with current practice, the ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer label does not include a statement under the heading "Pediatric Use" that appears in the GE Healthcare label as follows: "Safety and effectiveness in pediatric patients have not been established."
- Unlike the GE Healthcare label, the ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer label contains pediatric dosing information under the heading "How to Use ROTOP DMSA". Pediatric doses can also be calculated online through the Society of Nuclear Medicine and Molecular Imaging website's Pediatric Injected Activity Tool.
- The ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer label does not state the product is sterile; however, like the GE Healthcare product, ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer is manufactured to be sterile.
- Side effects encountered with use of the ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer within the U.S. can be reported directly to Theragnostics, Inc., at 1-888-286-3848 rather than the foreign site referenced in the label for ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer.
ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection is available only by prescription in the U.S.
Please refer to the package insert for the FDA-approved DMSA Kit for the Preparation of Technetium Tc99m Succimer drug product for full prescribing information.
ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (Theragnostics) does not contain a barcode. Institutions should manually input the product into their systems. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
If you have any questions about the information contained in this letter or the use of ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (Theragnostics), please contact Theragnostics, Inc., Braintree, Massachusetts, 1-617-286-7479, 9:00 AM to 5:00 PM Eastern time.
Please note the following information prior to placing an order for ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (Theragnostics). On September 1, 2020, GE Healthcare sold their radiopharmacy chain to a third party, Radioisotope Life Sciences (RLS), and no longer have the capability to make radiolabelled 99mTc-DMSA.
To place an order for ROTOP DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection (Theragnostics), please contact your local nuclear pharmacy, ex. Cardinal Health, Jubilant, RLS, UPPI, Pharmalogic or another independent nuclear pharmacy. You can also contact Medi-Physics Inc., dba GE Healthcare, 1-800-292-8514, 8:00 AM to 6:30 PM Eastern time; or via web portal address ci.gehealthcare.com/us.
To report adverse events or quality problems associated with the use of this product, please call Theragnostics, Inc., Braintree, Massachusetts, 1-888-286-3848
CONTACT NUMBERS: Please use the following contact numbers as appropriate:
Phone: 1-617-286-7479
Fax: 1-617-398-6337
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178)
Sincerely,
Gregory Mullen
President & CEO
Attachments:
1. Product Comparison Table
2. Label Comparison Table
3. Vial and Carton Labels
Attachment 1: Product Comparison Table
| Characteristics | Reference product: MPI DMSA KIDNEY REAGENT (Kit for the Preparation of Technetium Tc99m Succimer Injection) | Theragnostics' product: Kit for the Preparation of Technetium Tc99m Succimer Injection |
|---|---|---|
| Conditions of use | DMSA is indicated for the use as an aid in the scintigraphic evaluation of renal parenchymal disorders. | Theragnostics' Kit is indicated for the use as an aid in the scintigraphic evaluation of renal parenchymal disorders. |
| Active ingredient | meso-2,3-dimercaptosuccinic acid | meso-2,3-dimercaptosuccinic acid |
| Inactive ingredients | stannous chloride dihydrate | stannous chloride dihydrate |
| ascorbic acid | ascorbic acid | |
| inositol | --- | |
| sodium hydroxide | sodium hydroxide | |
| hydrochloric acid | hydrochloric acid | |
| nitrogen | nitrogen | |
| Route of Administration | Intravenous | Intravenous |
| Dosage form | Injection | Injection |
| Strength | N/A | N/A |
| Description | Each vial contains a sterile, pyrogen-free freeze-dried mixture of 1.0 mg dimercaptosuccinic acid, 0.42 mg stannous chloride dihydrate [0.38 mg (minimum) stannous chloride dihydrate (SnCl2 ∙2H2O) and 0.46 mg (maximum) total tin expressed as stannous chloride dihydrate (SnCl2 ∙2H2O)], 0.70 mg ascorbic acid, and 50.0 mg inositol. After freeze-drying, vials are sealed under a nitrogen atmosphere with a rubber closure. Sodium hydroxide and hydrochloric acid have been used for pH adjustment. When sterile, oxidant-free, pyrogen-free sodium pertechnetate Tc99m injection in isotonic saline is combined with the vial contents, following the instructions provided with the kit, a complex is formed. After 10 minutes' incubation the reconstituted solution is ready for intravenous injection. | One vial contains 1.74 mg powder with the active substance, 1.0 mg succimer. The excipients are: stannous chloride dihydrate, ascorbic acid, sodium hydroxide, hydrochloric acid 36% and nitrogen. |
Attachment 2: Labeling Comparison Table
| GE REFERENCE PRODUCT INSERT | DIFFERENCES | ROTOP-DMSA INSERT | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSA English translation note | This Package Leaflet and Summary of Product Characteristics was translated by the manufacturer based on the original German document (Vs. 4), authorized by the German Federal Institute for Drugs and Medicinal Services in November 2014. | |||||||||||||||||
| Package Leaflet and Summary of Product Characteristics | ||||||||||||||||||
| DMSA Kit for the Preparation of Technetium Tc99m Succimer Injection | Product name specific for market | ROTOP - DMSA, 1.0 mg Kit for radiopharmaceutical preparation Succimer |
||||||||||||||||
| DIAGNOSTIC - FOR INTRAVENOUS USE | Insert layout specific to manufacturer; GE layout adjusted to "line" up to sections with ROTOP insert for ease of review German product specific instructions | Read all of this leaflet carefully before you start using this medicine.
| ||||||||||||||||
In this leaflet:
|
||||||||||||||||||
| DESCRIPTION
Each vial contains a sterile, pyrogen-free freeze-dried mixture of 1.0 mg dimercaptosuccinic acid, 0.42 mg stannous chloride dihydrate [0.38 mg (minimum) stannous chloride dihydrate (SnCl2∙2H2O) and 0.46 mg (maximum) total tin expressed as stannous chloride dihydrate (SnCl2∙2H2O)], 0.70 mg ascorbic acid, and 50.0 mg inositol. After freeze-drying, vials are sealed under a nitrogen atmosphere with a rubber closure. Sodium hydroxide and hydrochloric acid have been used for pH adjustment. When sterile, oxidant-free, pyrogen-free sodium pertechnetate Tc99m injection in isotonic saline is combined with the vial contents, following the instructions provided with the kit, a complex is formed. After 10 minutes incubation the reconstituted solution is ready for intravenous injection. Chemical Name: meso-2,3-dimercaptosuccinic acid 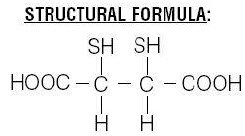 The succimer component of DMSA consists of more than 90% meso isomer and less than 10% d,l isomer. | Insert layout and details specific to manufacturer | 1. WHAT ROTOP – DMSA IS AND WHAT IT IS USED FOR
ROTOP - DMSA is a radiodiagnostic pharmaceutical. The kit contains the non-radioactive powder for reconstitution of the [99mTc]technetium succimer injection solution ([99mTc]-DMSA). The sodium [99mT]pertechnetat which is needed for the preparation is not part of this kit. After labelling with sodium [99mTc]technetium pertechnetat solution, ROTOP - DMSA is used for static renal scintigraphy when adequate diagnostics are not possible using other diagnostic procedures (such as ultrasound):
|
||||||||||||||||
| PHYSICAL CHARACTERISTICS
Technetium Tc99m decays by isomeric transition with a physical half-life of 6.02 hours1. The principal photon that is useful for detection and imaging studies is listed in Table 1. | Insert layout and details specific to manufacturer | |||||||||||||||||
| Table 1. Principal Radiation Emission Data1 | ||||||||||||||||||
| Radiation | Mean % / Disintegration | Mean Energy (keV) | ||||||||||||||||
| Gamma 2 | 89.07 | 140.5 | ||||||||||||||||
| 1 Kocher, David C., "Radioactive Decay Data Tables," DOE/TIC-11026,108 (1981). | ||||||||||||||||||
| INDICATIONS AND USAGE
DMSA is to be used as an aid in the scintigraphic evaluation of renal parenchymal disorders. PRECAUTIONS General As in the use of any radioactive material, care should be taken to minimize radiation exposure to the patient consistent with proper patient management and to ensure minimum radiation exposure to occupational workers. DMSA should be used between 10 minutes and 4 hours following reconstitution (see "Preparation" section). Any unused portion should be discarded after that time. Some patients with advanced renal failure may exhibit poor renal intake of Tc99m DMSA. It has been reported that satisfactory images may be obtained in some of these patients by delaying imaging for up to 24 hours. The contents of the kit vials are intended only for use in the preparation of DMSA Injection and are not to be directly administered to the patient. The contents of the kit vials are not radioactive. However, after Tc99m is added, adequate shielding of the final preparation must be maintained. Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. Carcinogenesis, Mutagenesis, Impairment of Fertility No long term animal studies have been performed to evaluate carcinogenic potential, mutagenic potential, or whether technetium Tc99m succimer injection affects fertility in males or females. Pregnancy Category C Animal reproduction studies have not been conducted with technetium Tc99m succimer injection. It is also not known whether technetium Tc99m succimer injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Technetium Tc99m succimer injection should be administered to a pregnant woman only if clearly needed. Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of child bearing capability should be performed during the first few (approximately 10) days following the onset of menses. Nursing Mothers Technetium Tc99m is excreted in human milk during lactation; therefore, formula feedings should be substituted for breast feedings. Pediatric Use Safety and effectiveness in pedriatric patients have not been established. Geriatric Use Clinical studies of DMSA did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | Insert layout and details specific to manufacturer | 2. BEFORE YOU USE ROTOP - DMSA Take special care with ROTOP – DMSA ROTOP - DMSA is not suitable for determining global renal function from the DMSA accumulation. In the case of proximal tubulopathies [99mTc]DMSA does not lead to a sufficient diagnostic renal accumulation. The patient must be well hydrated before and after administration. In order to keep radiation exposure to a minimum, patients must be encouraged to empty their bladders as often as possible during the first hours after the examination. For each patient it should be carefully considered whether the expected diagnostic benefits outweigh the risk linked to radiation exposure. In order to keep the radiation dose as low as possible, the administered activity may not be higher than that required for eliciting the diagnostic information. Radiopharmaceuticals may be received, used and administered only by authorised persons in areas specially designated for this purpose. The manipulation and use of these products is subject to the regulations of the local supervisory authority and/or requires appropriate permission. Contraindications ROTOP-DMSA should not be used in case of hypersensitivity to the active substance or to any of the excipients listed in section 6. Using other medicines Chemotherapeutic agents such as methotrexate, cyclophosphamide and vincristine can alter the biodistribution of [99mTc]DMSA. Shifting the acid/base balance, e.g. through ammonium chloride or sodium hydrogen carbonate, effects in vivo a change in the valence of the [99mTc]DMSA complex and in turn a lower accumulation in the renal cortex with simultaneous strong accumulation in the liver and rapid urine excretion. Mannitol leads to dehydration and in turn to a reduction in the extraction of [99mTc]DMSA. In the case of renal artery stenosis, ACE inhibitors can lead to a reversible insufficiency of the tubular function and in turn to a reduced accumulation of [99mTc]DMSA as a result of the reduction in filtration pressure in the affected kidney. If high doses of other chelating agents are injected at the same time, the stability of the [99mTc]DMSA DMSA may be influenced, thus effecting a change in kinetics. Pregnancy and lacation Pregnancy: No data on the clinical use of [99mTc]DMSA with pregnant women is available. If it is necessary to administer a radiopharmaceutical product to a woman of child-bearing age, she must have a pregnancy test first. If a woman has missed a period, it must be assumed that she is pregnant. In case of doubt, radiation exposure must be reduced to the minimum amount required to acquire the needed clinical information. In this case, alternative investigative methods must be considered that do not use ionising radiation. Radiopharmaceutical examinations of pregnant women also expose the foetus to radiation. For this reason, [99mTc]DMSA may only be used if there is a vital indication and if the expected benefit outweighs the risk to mother and child. Lactation: Before administering [99mTc]DMSA to a breast-feeding mother, it must be considered whether the investigation could also be delayed until the mother has ceased breast-feeding and as to whether using a radiopharmaceutical is the most appropriate examination method, bearing in mind the secretion of activity into breast milk. If administering [99mTc]DMSA is deemed necessary, breast-feeding must be interrupted for at least 12 hours, and the expressed breast milk discarded. |
||||||||||||||||
| Insert layout and details specific to manufacturer | Driving and using machines
Effects on the ability to drive or use machines have not been described. |
|||||||||||||||||
| DOSAGE AND ADMINISTRATION
The suggested dose range for slow I.V. administration to be employed in the average patient (70 kg) for renal parenchymal imaging is 74-222 MBq, 2-6 mCi technetium Tc99m succimer injection. The product must be used between 10 minutes to 4 hours following preparation (see "Preparation" section). Acceptable renal images may be obtained beginning 1 to 2 hours post injection. Any unused portion should be discarded after that time. The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration. Do not use after the expiration date stated on the label. The components of the kit are supplied sterile and pyrogen-free. Aseptic procedures normally employed in making additions and withdrawals from sterile, pyrogen-free containers should be used during addition of sodium pertechnetate Tc99m injection solutions and during the withdrawal of doses for patient administration. | Precautions for avoiding hazards for the environment
Radiopharmaceuticals must be prepared and used by the user under precautions for the protection from ionizing radiation and taking pharmaceutical quality standards into account. In accordance with the guidelines for Good Pharmaceutical Manufacturing Practice, work must be done under aseptic conditions. Patients treated with radiopharmaceuticals pose a risk for other persons based on external radiation exposure or contamination due to spilling urine, vomiting, etc. For this reason, the precautionary measures provided by the national radiation protection regulations must be observed. Contamination brought about by radioactivity that has been excreted by the patient must be avoided. 3. HOW TO USE ROTOP - DMSA Single intravenous use after preparation with sodium [99mTc]pertechnetate solution. Adults are given 0.3 to 1.0 mg succimer and activities of 70 MBq. Scintigraphic examinations should not be carried out until at least 1 hour after application; waiting 3 hours is preferable. In the case of very poor renal function, waiting periods of up to 6 hours should be observed. The patient must be well hydrated. Children The recommendation of the Paediatric Task Group of the European Association of Nuclear Medicine (EANM) of 1990 lists the paediatric dose scaled to body weight as a fraction of the adult dose: |
|||||||||||||||||
| Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. | 3 kg = 0.1 | 22 kg = 0.50 | 42 kg = 0.78 | |||||||||||||||
| 4 kg = 0.14 | 24 kg = 0.53 | 44 kg = 0.80 | ||||||||||||||||
| 6 kg = 0.19 | 26 kg = 0.56 | 46 kg = 0.82 | ||||||||||||||||
| 8 kg = 0.23 | 28 kg = 0.58 | 48 kg = 0.85 | ||||||||||||||||
| 10 kg = 0.27 | 30 kg = 0.62 | 50 kg = 0.88 | ||||||||||||||||
| 12 kg = 0.32 | 32 kg = 0.65 | 52 - 54 kg = 0.90 | ||||||||||||||||
| 14 kg = 0.36 | 34 kg = 0.68 | 56 - 58 kg = 0.92 | ||||||||||||||||
| Insert layout and details specific to manufacturer | 16 kg = 0.40 | 36 kg = 0.71 | 60 - 62 kg = 0.96 | |||||||||||||||
| 18 kg = 0.44 | 38 kg = 0.73 | 64 - 66 kg = 0.98 | ||||||||||||||||
| 20 kg = 0.46 | 40 kg = 0.76 | 68 kg = 0.99 | ||||||||||||||||
| WARNINGS
None. ADVERSE REACTIONS Rare instances of syncope, fever, nausea and maculopapular skin rash have been reported. CONTRAINDICATIONS None known. | Activity of less than 20 % (15 MBq) of the adult dose generally does not allow a satisfactory assessment to be derived from the examination. If you use more ROTOP – DMSA than you should Due to the low amounts of substances used, overdosage in the pharmacological sense is not expected. Exposure to radiation resulting from an overdosage of radioactivity can be reduced by forced diuresis. 4. POSSIBLE SIDE EFFECTS As all medicinal products, ROTOP - DMSA can cause side effects, although not everybody gets them. For assessing the side effects the frequency is classified as follows: |
|||||||||||||||||
| Very common | observed in more than 1 patients in 10 | |||||||||||||||||
| Common | observed in less than 1 patient in 10, but more than 1 patient in 100 | |||||||||||||||||
| Uncommon | observed in less than 1 patient in 100, but more than 1 patient in 1,000 | |||||||||||||||||
| Rare | observed in less than 1 patient in 1,000, but more than 1 patient in 10,000 | |||||||||||||||||
| Very rare | observed in less than 1 patient in 10,000 or not known | |||||||||||||||||
| In very rare cases (< 0.01 %) after intravenous injection of the ready-to-use solution, hypersensitivity reactions have occurred such as locally confined or general rashes, itching, drop in blood pressure, headache, dizziness, nausea and vomiting. Reactions can occur up to 24 hours after the injection. Although such reactions are very rare and usually very minor, appropriate instruments and medications for immediate treatment of allergic reactions (adrenaline, corticosteroids and antihistamines) should be within reach for possible emergency treatment at all times. |
||||||||||||||||||
| Insert layout and details specific to manufacturer | Since the administered amounts of active substances are very low, the risks of use are mainly related to radiation exposure. Ionising radiation can cause cancer and genetic mutations. Since most radiopharmaceutical examinations are conducted with low effective radiation doses of less than 20 mSv, the probability of such effects occurring is expected to be low. |
|||||||||||||||||
| HOW SUPPLIED Kit Contents 5 Vials containing a freeze-dried mixture of 1.0 mg dimercaptosuccinic acid, 0.42 mg stannous chloride dihydrate [0.38 mg (minimum) stannous chloride dihydrate (SnCl2∙2H2O) and 0.46 mg (maximum) total tin expressed as stannous chloride dihydrate (SnCl2∙2H2O)], 0.70 mg ascorbic acid, and 50.0 mg inositol. 5 Labels 1 Package Insert | The effective radiation dose is 0.62 mSv when the maximum recommended activity of this medicinal product is applied. Reporting of side effects If you notice any side effects please contact your nuclear physician responsible for supervising the administration. This also applies to any side effects not listed in this leaflet. You can also report any side effects directly to: Bundesinstitut für Arzneimittel und Medizinprodukte, Abt. Pharmakovigilanz, Kurt-Georg-Kiesinger Allee 3, D-53175 Bonn, website: http://www.bfarm.de. By reporting side effects you can help provide more information on the safety of this medicine. 5. HOW TO STORE ROTOP - DMSA Keep out of the reach and sight of children. Do not use this medicinal product after the expiry date stated on the label. |
|||||||||||||||||
| NDC 017156-525-01 Storage Store the kit at 2°-8°C (36°-46°F) and protect from light. | Storage conditions
Store refrigerated (2 to 8 °C) in the original package. Radiopharmaceuticals must be stored in accordance with the regulations for radioactive protection and in particular be kept from unauthorised access. Shelf life after opening and reconstitution The product labelled with [99mTc]technetium can be injected within 4 hours after reconstitution and has to be stored at room temperature (15–25 °C) during this time. 6. FURTHER INFORMATION What ROTOP – DMSA contains One vial contains 1.74 mg powder with the active substance: 1.0 mg succimer |
|||||||||||||||||
| This reagent kit is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant to 32 Ill. Code Adm. Section, Section 330.260(a) and 335.4010 or under equivalent licenses of the U.S. Nuclear Regulatory Commission, or an Agreement State. | Insert layout and details specific to manufacturer | The other ingredients are: Stannous chloride dihydrate Ascorbic acid Sodium hydroxide Hydrochloric acid 36% Nitrogen |
||||||||||||||||
| Manufactured for: GE Healthcare Medi-Physics, Inc. 3350 North Ridge Avenue Arlington Heights, IL 60004 1-800-633-4123 (Toll Free) By: GE Healthcare Ltd. Little Chalfont, HP7 9NA, UK GE and the GE Monogram are trademarks of General Electric Company. 43-4349H L/2331/04 Revised February 2006 | What ROTOP – DMSA looks like and contents of the pack:
The package consists of a carton with 5 vials. Marketing Authorisation Holder and Manufacturer ROTOP Pharmaka GmbH, Bautzner Landstr. 400, 01328 Dresden, Germany Tel: 0049 + (0) 351 – 26 310 210 Fax: 0049 + (0) 351 – 26 310 313 e-mail: service@rotop-pharmaka.de This medicinal product is authorised in the Member States of the EEA under the following names: |
|||||||||||||||||
| Germany: | ROTOP - DMSA | |||||||||||||||||
| CLINICAL PHARMACOLOGY | This leaflet was last approved in May 2017. | |||||||||||||||||
| After intravenous administration, technetium Tc99m succimer injection is distributed in the plasma, apparently bound to plasma proteins. | ||||||||||||||||||
| There is negligible activity in the red blood cells. The activity is cleared from the plasma with a half-time of about 60 minutes and concentrates in the renal cortex. Approximately 16% of the activity is excreted in the urine within two hours. At six hours about 20% of the dose is concentrated in each kidney. | The following information is intended for medical or healthcare professionals only: | |||||||||||||||||
| EXTERNAL RADIATION
The specific gamma ray constant for technetium Tc99m is 0.78 R/hr-mCi at 1 cm. The first half value layer is 0.017 cm of Pb. To facilitate control of the radiation exposure from millicurie amounts of this radionuclide, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000. | PHARMACOLOGICAL PROPERTIES Pharmacodynamic properties Pharmacotherapeutic group: Diagnostic radiopharmaceutical for renal diagnostics (ATC: V09CA02). Based on current research, for the low amounts of substances used for imaging techniques no clinically relevant pharmacodynamic effects of [99mTc]DMSA are expected. |
|||||||||||||||||
| Table 2. Radiation Attenuation by Lead Shielding | Pharmacokinetic properties
After intravenous injection, within 5 minutes over 70% of the [99mTc]DMSA is bound to theα-2 microglobulin fraction in blood plas ma. Binding to erythrocytes may be disregarded. One hour post injection, 25% of the radiopharmaceutical is already located in the renal cortex and only 30% remains in the plasma. Approx. 10% appears in the urine. In healthy persons, the plasma clearance of [99mTc]DMSA amounts to approx. 10 ml/min. (scaled to 1.73 sqm body surface). After approx. 3 hours, the maximum renal accumulation is reached. In healthy persons, at this point approx. 50% of the radiopharmaceutical is located in the renal cortex, approx. 20% remains in the plasma and just under 10% in the liver and muscles. Within 24 hours, approx. 30% is excreted with the urine. |
|||||||||||||||||
| Shield Thickness (Pb) cm | Coefficient of Attenuation | |||||||||||||||||
| 0.02 | 0.5 | Insert layout and details specific to manufacturer | ||||||||||||||||
| 0.08 | 0.1 | |||||||||||||||||
| 0.16 | 0.01 | |||||||||||||||||
| 0.25 | 0.001 | |||||||||||||||||
| 0.33 | 0.0001 | |||||||||||||||||
| To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 3. | ||||||||||||||||||
| Table 3. Physical Decay Chart: Tc99m, half-life 6.02 hours | [99mTc]DMSA accumulates in the pars recta and convoluta of the proximal renal tubules – most likely due to peritubular reabsorption. On an intracellular level, the majority of the [99mTc]DMSA is bound to a soluble protein in the cytosol. This mechanism, which has not yet been explained in detail, is disrupted in the case of proximal tubulopathies (such as nephritides or the Fanconi syndrome), which can be recognised by the increased plasma clearance of [99mTc]DMSA and low renal accumulation. | |||||||||||||||||
| Hours | Fraction Remaining | Hours | Fraction Remaining | |||||||||||||||
| 0* | 1.000 | 7 | 0.447 | |||||||||||||||
| 1 | 0.891 | 8 | 0.398 | |||||||||||||||
| 2 | 0.794 | 9 | 0.355 | |||||||||||||||
| 3 | 0.708 | 10 | 0.316 | |||||||||||||||
| 4 | 0.631 | 11 | 0.282 | |||||||||||||||
| 5 | 0.562 | 12 | 0.251 | |||||||||||||||
| 6 | 0.501 | |||||||||||||||||
| * Calibration Time | ||||||||||||||||||
| DISPOSAL
Any unused portion of the Tc99m-labeled kit must be stored and disposed of in accordance with the conditions of NRC radioactive materials license pursuant to 10 CFR Parts 20 and 35 or equivalent conditions pursuant to Agreement state regulation, or other regulatory agency authorized to license the use of radionuclides. The unlabeled residual materials may be discarded in ordinary trash, provided that the vials and syringes read background with an appropriate low-range survey meter. It is suggested that all identification labels be destroyed before discarding. RADIATION DOSIMETRY The estimated absorbed radiation doses2,3 to an average adult (70 kg) are shown in Table 4. | Toxicological properties
Due to the low amounts of DMSA and stannous chloride contained in the kit, toxic effects brought about by the substances are not expected if used according to directions. Data on investigations on reproduction toxicity as well as on mutagenicity and cancerogenity are not available. Special precautions for disposal and further directions for handling The empty package is considered to be regular waste if the permitted level for [99mTc]technetium is not exceeded (≤ 0.5 Bq/g or 0.5 Bq/cm2). Particulars indicating radioactivity must be removed prior to disposing of the non-radioactive waste and must be destroyed separately. Radioactive waste must be disposed of as provided by law. MARKETING AUTHORISATION NUMBER 3003663.00.00 DATE OF FIRST AUTHORISATION/ RENEWAL OF THE AUTHORISATION 24/11/2005 |
|||||||||||||||||
| Table 4. Absorbed Radiation Dose | Insert layout and details specific to manufacturer | DOSIMETRY Radiation exposure According ICRP publication 80 (Table 1) the following radiation doses will be absorbed: |
||||||||||||||||
| Tissue | mGy / 222 MBq | rads / 6 mCi | ||||||||||||||||
| Bladder Wall | 4.2 | 0.42 | ||||||||||||||||
| Kidneys (total) | 37.8 | 3.78 | ||||||||||||||||
| Renal Cortices | 51.0 | 5.10 | ||||||||||||||||
| Liver | 1.9 | 0.19 | ||||||||||||||||
| Bone Marrow | 1.3 | 0.13 | ||||||||||||||||
| Ovaries | 0.8 | 0.08 | ||||||||||||||||
| Testes | 0.4 | 0.04 | ||||||||||||||||
| Total Body | 0.9 | 0.09 | ||||||||||||||||
| Absorbed dose per unit of activity administered (mGy/MBq) | ||||||||||||||||||
| 2 Method of Calculation: A schema for Absorbed-Dose Calculations for Biologically Distributed Radionuclides, Supplement No. 1, MIRD Pamphlet No. 1, J. Nucl. Med., p. 7, 1968. 3 Biological Data: Arnold, R.W; Subramanian, G.; McAfee, J.G.; Blair, R.J.; Thomas, F.D.; Comparison of Tc99m complexes for renal imaging, J. Nucl. Med., 16, pp. 357-367, 1975. | Organ | Adults | 15 years | 10 years | 5 years | 1 year | ||||||||||||
| Adrenals | 0.012 | 0.016 | 0.024 | 0.035 | 0.060 | |||||||||||||
| Bladders wall | 0.018 | 0.023 | 0.029 | 0.031 | 0.057 | |||||||||||||
| Bone surface | 0.0050 | 0.0062 | 0.0092 | 0.014 | 0.026 | |||||||||||||
| Brain | 0.0012 | 0.0015 | 0.0025 | 0.0040 | 0.0072 | |||||||||||||
| Breast | 0.0013 | 0.0018 | 0.0028 | 0.0045 | 0.0084 | |||||||||||||
| Gall bladder | 0.0083 | 0.010 | 0.014 | 0.022 | 0.031 | |||||||||||||
| Stomach wall | 0.0052 | 0.0063 | 0.010 | 0.014 | 0.020 | |||||||||||||
| Colon | 0.0050 | 0.0063 | 0.010 | 0.014 | 0.024 | |||||||||||||
| Intestine | 0.0043 | 0.0055 | 0.0082 | 0.012 | 0.020 | |||||||||||||
| Upper large intestine | 0.0050 | 0.0064 | 0.095 | 0.014 | 0.023 | |||||||||||||
| Lower large intestine | 0.0035 | 0.0043 | 0.0065 | 0.0096 | 0.016 | |||||||||||||
| Heart | 0.0030 | 0.0038 | 0.0058 | 0.0086 | 0.014 | |||||||||||||
| Kidneys | 0.18 | 0.22 | 0.30 | 0.43 | 0.76 | |||||||||||||
| Liver | 0.0095 | 0.012 | 0.018 | 0.025 | 0.041 | |||||||||||||
| Lungs | 0.0025 | 0.0035 | 0.0052 | 0.0080 | 0.015 | |||||||||||||
| Muscles | 0.0029 | 0.0036 | 0.0052 | 0.0077 | 0.014 | |||||||||||||
| Oesophagus | 0.0017 | 0.0023 | 0.0034 | 0.0054 | 0.0094 | |||||||||||||
| Ovaries | 0.0035 | 0.0047 | 0.0070 | 0.011 | 0.019 | |||||||||||||
| Pancreas | 0.0090 | 0.011 | 0.016 | 0.023 | 0.037 | |||||||||||||
| Red marrow | 0.0039 | 0.0047 | 0.0068 | 0.0090 | 0.014 | |||||||||||||
| Skin | 0.0015 | 0.0018 | 0.0029 | 0.0045 | 0.0085 | |||||||||||||
| Spleen | 0.013 | 0.017 | 0.026 | 0.038 | 0.061 | |||||||||||||
| Testes | 0.0018 | 0.0024 | 0.0037 | 0.0053 | 0.010 | |||||||||||||
| Thymus | 0.0017 | 0.0023 | 0.0034 | 0.0054 | 0.0094 | |||||||||||||
| Thyroid | 0.0015 | 0.0019 | 0.0031 | 0.0052 | 0.0094 | |||||||||||||
| Uterus | 0.0045 | 0.0056 | 0.0083 | 0.011 | 0.019 | |||||||||||||
| Remaining organ | 0.0029 | 0.0037 | 0.0052 | 0.0077 | 0.014 | |||||||||||||
| Insert layout and details specific to manufacturer | Effective Dose per unit of activity administered (mSv/MBq) | 0.0088 | 0.011 | 0.015 | 0.021 | 0.037 | ||||||||||||
| In an adult (70 kg), after intravenous injection of 70 MBq (maximum dose) [99mTc]DMSA, the effective dose is approx. 0.62 mSv. The absorbed dose in the target organ kidney is approx. 12.6 mGy and in the critical organ bladder wall 1.26 mGy. | ||||||||||||||||||
| Radiophysical Properties | ||||||||||||||||||
| Preparation
The following directions must be carefully followed for optimum preparation of technetium Tc99m succimer injection: Note: Use aseptic procedures throughout and take precautions to minimize radiation exposure by the use of suitable shielding. Waterproof gloves should be worn during the preparation procedure.
| Insert layout and details specific to manufacturer | [99mTc]technetium is produced using a [99Mo/99mTc] sterile generator and decays releasing gamma radiation with an energy of 140/142 keV with a half-life of 6.02 hours to [99Tc]technetium, which in turn decays to stable [99Ru]ruthenium; However, due to a long half-life of 214,000 years, 99Tc itself is considered to be stable. INSTRUCTIONS FOR PREPARATION OF RADIOPHARMACEUTICALS Instruction for labelling [99mTc]technetium succimer injection solution is prepared under sterile conditions with a sodium [99mTc]pertechnetate injection solution (European Pharmacopoeia quality 4.00/0124 or 4.00/0283) directly before use. Oxygenation must be avoided. Place the vial with powder in sufficient lead shielding with ample space and disinfect the stopper (allow disinfectant to dry). Use a syringe with the smallest possible cannula lumen to transfer 5 mL sodium [99mTc]technetium pertechnetate solution with a maximum of 3 GBq to the vial. Use the same syringe to withdraw the appropriate gas volume from the vial for pressure compensation. Lightly shake the vial in order to completely dissolve the powder. The stopper should be well moistened as well. After 10 minutes reaction time, measure the overall activity. If needed, the finished injection solution can be diluted with sterile isotonic sodium chloride to a total volume of up to 10 mL. Quality Control Prior to use in the patient, the radiochemical purity of the [99mTc]technetium succimer injection solution must be tested using the method described below: |
||||||||||||||||
| Rx ONLY | Preparation:
Type of test: Thin layer chromatography Plates used: Silica gel on a glass fibre plate, heated for 10 min. at 110 °C prior to testing Starting point: 1.5 cm from lower end of the plate Migration distance: 10 to 15 cm (in approx. 15 minutes) Execution: Use a capillary tube or pipette to extract a volume of approx. 5 μl and apply it to the plate. Chromatography begins immediately with a solution of methylethylketone (MEK) over a migration distance of 10 to 15 cm. Allow the plate to air-dry, and use a detector to determine the distribution of radioactivity. Evaluation: The [99mTc] technetium succimer complex remains at the starting point while [99mTc] pertechnetate migrates near the solvent front. |
|||||||||||||||||
| Target value: | ≥ 95.0 % [99mTc]technetium succimer | |||||||||||||||||
| ≤ 2.0% [99mTc]pertechnetate | ||||||||||||||||||
| CLASSIFICATION FOR SUPPLY
Pharmacy-only medicine |
||||||||||||||||||
Attachment 3: Product Labels
Vial
Carton

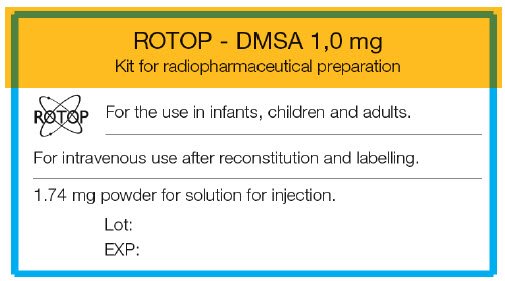
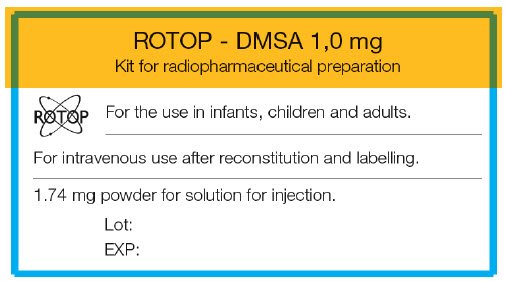
PRINCIPAL DISPLAY PANEL - 1.74 mg Vial Label
ROTOP - DMSA 1,0 mg
Kit for radiopharmaceutical preparation
ROTOP
For the use in infants, children and adults.
For intravenous use after reconstitution and labelling.
1.74 mg powder for solution for injection.
Lot:
EXP:
PRINCIPAL DISPLAY PANEL - 5 Vial Carton Label
ROTOP – DMSA 1.0 mg
Kit for radiopharmaceutical preparation
Succimer
For the use in infants, children and adults.
5 vials
Content/vial:
1.74 mg powder for solution for injection
active substance: 1.0 mg succimer
excipients: stannous chloride dihydrate, ascorbic acid, sodium
hydroxide, hydrochloric acid, nitrogen
For intravenous use after reconstitution and labelling. Store in the original package in order
to protect from light. Store in a refrigerator at 2 – 8 °C. Keep out of the sight and reach of
children.
MA Number: 3003663.00.00
pharmacy only medicine
ROTOP Pharmaka GmbH, Bautzner Landstraẞe 400, 01328 Dresden, Germany
ROTOP

| ROTOP - DMSA
kit for the preparation of technetium tc99m succimer injection injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ROTOP Pharmaka GmbH (314666202) |
| Registrant - Theragnostics Inc. (080437847) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ROTOP Pharmaka GmbH | 314666202 | MANUFACTURE(71647-001) | |
