Posatex Otic Suspension: Package Insert / Prescribing Info
Package insert / product label
Generic name: orbifloxacin, mometasone furoate, and posaconazole otic Suspension
Dosage form: FOR ANIMAL USE ONLY
On This Page
Antibacterial, anti-inflammatory, antifungal
For Otic Use in Dogs Only
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Federal law prohibits the extralabel use of this drug in food-producing animals.
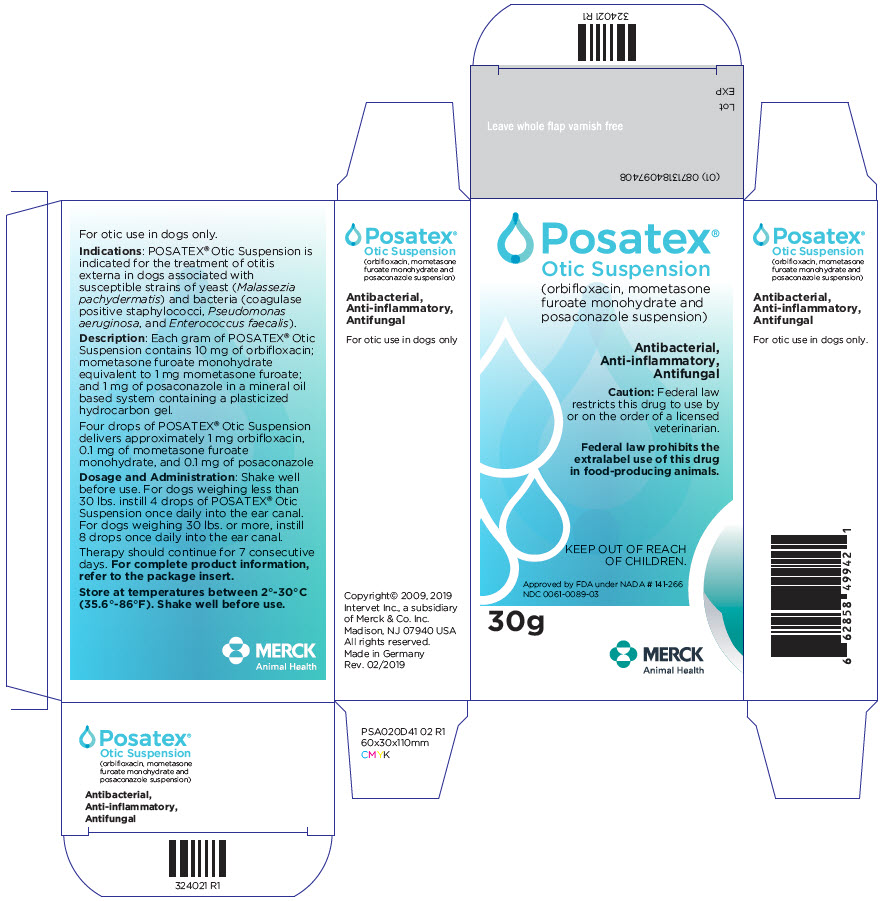
DESCRIPTION: Each gram of POSATEX® Otic Suspension contains 10 mg of orbifloxacin; mometasone furoate monohydrate equivalent to 1 mg mometasone furoate; and 1 mg of posaconazole in a mineral oil based system containing a plasticized hydrocarbon gel.
Four drops of POSATEX® Otic Suspension delivers approximately 1.0 mg orbifloxacin, 0.1 mg of mometasone furoate monohydrate, and 0.1 mg of posaconazole.
INDICATIONS: POSATEX® Otic Suspension is indicated for the treatment of otitis externa in dogs associated with susceptible strains of yeast (Malassezia pachydermatis) and bacteria (coagulase positive staphylococci, Pseudomonas aeruginosa, and Enterococcus faecalis).
DOSAGE AND ADMINISTRATION: Shake well before use. For dogs weighing less than 30 lbs. instill 4 drops of POSATEX® Otic Suspension once daily into the ear canal. For dogs weighing 30 lbs. or more, instill 8 drops once daily into the ear canal. Therapy should continue for 7 consecutive days.
CONTRAINDICATIONS: POSATEX® Otic Suspension is contraindicated in dogs with known or suspected hypersensitivity to quinolones, mometasone furoate monohydrate, or posaconazole. Do not use in dogs with known tympanic perforation (see PRECAUTIONS).
Warnings
Animal Warnings: Do not administer orally. Immediately discontinue use of POSATEX® Otic Suspension if hearing loss is observed during treatment (see ADVERSE REACTIONS).
PRECAUTIONS: The use of POSATEX® Otic Suspension in dogs with perforated tympanic membranes has not been evaluated. The integrity of the tympanic membranes should be confirmed before administering this product.
Avoid prolonged or repeated use of POSATEX® Otic Suspension. Long-term use of topical otic corticosteroids has been associated with adrenocortical suppression and iatrogenic hyperadrenocorticism in dogs (see ANIMAL SAFETY).
The safe use of POSATEX® Otic Suspension in dogs used for breeding purposes, during pregnancy or in lactating bitches, has not been evaluated. The systemic administration of quinolones has been shown to produce cartilage erosions of weight bearing joints and other signs of arthropathy in immature animals of various species.
ADVERSE REACTIONS: In the field study, 143 dogs were treated with POSATEX® Otic Suspension. Of those, 1 dog with bilateral otitis externa developed hearing loss. POSATEX® Otic Suspension treatment was discontinued and the condition resolved after one week.
To report suspected adverse reactions, call 1-800-224-5318.
For a copy of the Material Safety Data Sheet (MSDS) call 1-800-770-8878.
Posatex Otic Suspension - Clinical Pharmacology
Orbifloxacin: Orbifloxacin is a synthetic fluoroquinolone antibacterial agent. The bactericidal action of fluoroquinolones is concentration-dependent and results from interference with bacterial DNA gyrase and topoisomerase IV. Since these enzymes are needed for bacterial DNA synthesis and transcription, fluoroquinolones disrupt bacterial replication and lead to bacterial cell death.
Mometasone: Mometasone furoate monohydrate is a topical corticosteroid characterized by a (2') furoate 17-ester having chlorine at the 9 and 21 positions.
Posaconazole: Posaconazole is a broad-spectrum triazole antifungal agent. The mechanism by which triazoles exert fungicidal action involves the selective inhibition of the enzyme lanosterol a C14 demethylase (a microsomal cytochrome P-450- dependent enzyme) involved in ergosterol biosynthesis in yeasts and filamentous fungi.
Systemic absorption of the active ingredients was determined in single-dose radiolabelled studies with 14C-orbifloxacin, 3H-mometasone furoate, and 14C-posaconazole contained within the POSATEX® Otic Suspension formulation and placed into the ear canals of normal beagle dogs. Most of the absorption occurred in the first few days after administration. The extent of percutaneous absorption of topical medications is influenced by many factors including the integrity of the epidermal barrier. Inflammation can increase the percutaneous absorption of drugs.
EFFECTIVENESS: The effectiveness of POSATEX® Otic Suspension was evaluated in a placebo-controlled, double-blind, multi-site field study. One hundred and ninety one dogs with naturally occurring clinical otitis externa associated with both yeast and bacteria were randomly allocated to either POSATEX® Otic Suspension or placebo ointment. Of the 160 dogs evaluated for effectiveness, 122 were treated with POSATEX® Otic Suspension and 38 were treated with placebo ointment. Treatments were administered once daily for 7 consecutive days. Assessment of effectiveness was based on improvement in clinical signs at re-evaluation 2-7 days following administration of the last dose.
Compared to the placebo, a significant percent of dogs treated with POSATEX® Otic Suspension showed improvement in clinical signs (discomfort, erythema, and swelling) caused by otitis externa associated with one or more of the following organisms: Malassezia pachydermatis, coagulase positive staphylococci, Pseudomonas aeruginosa, and Enterococcus faecalis.
| Clinical Sign | POSATEX® Otic Suspension Group | Placebo Group | Significance |
|---|---|---|---|
| Discomfort | 88% | 45% | p<0.0001 |
| External Ear Canal Erythema | 81% | 39% | p<0.0001 |
| External Ear Canal Swelling | 83% | 49% | p=0.0001 |
ANIMAL SAFETY: POSATEX® Otic Suspension was administered at 1,3, and 5 times the recommended dosage for 21 consecutive days. The control group received the vehicle in both ears at the clinical dose given five times per day. There was a slight decrease in serum cortisol concentration after ACTH stimulation on Day 21 in the 5× group. Erythema was noted in all groups. Aural pain, swelling, or heat were each noted in 3 separate dogs in the 5× group.
Approved by FDA under NADA # 141-266
Copyright© 2009, 2019 Intervet Inc., a subsidiary of Merck & Co. Inc.
Madison, NJ 07940 USA
All rights reserved.
Made in Germany
Rev. 02/2019
PRINCIPAL DISPLAY PANEL - 30 g Bottle Label
Posatex®
Otic Suspension
(orbifloxacin, mometasone
furoate monohydrate and
posaconazole suspension)
Antibacterial,
Anti-inflammatory,
Antifungal
Caution: Federal law
restricts this drug to use by
or on the order of a licensed
veterinarian.
Federal law prohibits the
extralabel use of this drug
in food-producing animals.
KEEP OUT OF REACH
OF CHILDREN.
Approved by FDA under NADA # 141-266
NDC 0061-0089-03
30g
MERCK
Animal Health
| POSATEX
orbifloxacin, mometasone furoate, and posaconazole suspension |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme Corp. (001317601) |
