Papzimeos: Package Insert / Prescribing Info
Package insert / product label
Generic name: zopapogene imadenovec
Dosage form: injection, suspension
Medically reviewed by Drugs.com. Last updated on Sep 19, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
PAPZIMEOS (zopapogene imadenovec-drba) suspension for subcutaneous injection
Initial U.S. Approval: 2025
Indications and Usage for Papzimeos
PAPZIMEOS™ is a non-replicating adenoviral vector-based immunotherapy indicated for the treatment of adults with recurrent respiratory papillomatosis. (1)
Papzimeos Dosage and Administration
PAPZIMEOS is for subcutaneous injection only. (2.1)
The recommended dose of PAPZIMEOS is 5×1011 particle units (PU) per injection administered by subcutaneous injection four (4) times over a 12-week interval. (2.1)
Prior to the initial administration of PAPZIMEOS, perform a surgical debulking of visible papilloma to establish minimal residual disease. To maintain minimal residual disease during treatment with PAPZIMEOS, remove visible papilloma, if present, prior to the third and fourth administration of PAPZIMEOS. (2.1)
Dosage Forms and Strengths
PAPZIMEOS is supplied in a single-dose vial that contains 5×1011 PU in an extractable volume of 1 mL of suspension. (3)
Contraindications
None. (4)
Warnings and Precautions
- Injection-site reactions: Injection-site reactions, have been observed. Monitor patients for local site reactions for at least 30 minutes after the initial treatment. (5.1)
- Thrombotic events: Thrombotic events may occur following administration of adenoviral vector-based therapies. Monitor patients for signs and symptoms of thrombotic events and treat events according to clinical practice. (5.2)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥5%) were injection site reactions, fatigue, chills, pyrexia, myalgia, nausea, headache, tachycardia, diarrhea, vomiting, and hyperhidrosis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Precigen Inc. at 855-743-6777 and medinfo@precigen.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2025
Full Prescribing Information
1. Indications and Usage for Papzimeos
PAPZIMEOS is indicated for the treatment of adults with recurrent respiratory papillomatosis.
2. Papzimeos Dosage and Administration
2.1 Recommended Dosage
PAPZIMEOS is for subcutaneous injection only.
The recommended dosage of PAPZIMEOS is 5×1011 particle units (PU) per injection administered as subcutaneous injection four times over a 12-week interval. The recommended dosing schedule for PAPZIMEOS is shown in Table 1.
| Administration | Administration Interval |
|---|---|
|
1The second administration should occur no less than 11 days after the initial administration. |
|
| Initial | -- |
| Second | 2 weeks after initial administration1 |
| Third | 6 weeks after initial administration |
| Fourth | 12 weeks after initial administration |
Prior to the initial administration of PAPZIMEOS, perform a surgical debulking of visible papilloma to establish minimal residual disease. To maintain minimal residual disease during treatment with PAPZIMEOS, remove visible papilloma, if present, prior to the third and fourth administration of PAPZIMEOS.
2.2 Preparation and Handling
PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy. Follow universal biosafety precautions for handling.
PAPZIMEOS is provided as a single-dose vial of sterile frozen suspension.
PAPZIMEOS MUST BE RAPIDLY thawed before use and prepared for immediate administration.
Once thawed, DO NOT place the PAPZIMEOS vial in a refrigerator, freezer, or on dry ice. Protect PAPZIMEOS from light. DO NOT shake the vial.
Recommended Supplies and Materials
- Freezer for storage of PAPZIMEOS at temperature ≤ -60°C [≤ -76°F]
- Water bath or dry bead bath set to 37°C [98.6°F]
- 3 mL sterile syringe
- Sterile needle (18G to 22G without a filter) to withdraw PAPZIMEOS from the vial
- Sterile needle for subcutaneous injection (23G to 25G needle, 1/2 - 5/8 inch long)
- 70% isopropyl alcohol pads
Receipt of PAPZIMEOS
PAPZIMEOS is provided as a sterile, frozen suspension that has been aseptically filled into single-dose vials fitted with a rubber stopper and aluminum flip-cap seal. Each vial is sealed inside a pouch, which is placed in the carton along with a Package Insert. The PAPZIMEOS carton is shipped frozen at ≤ -60°C [≤ -76°F] in an insulated shipping box containing dry ice. On receipt, the PAPZIMEOS carton must be stored in a freezer at ≤ -60°C [≤ -76°F].
Preparation of PAPZIMEOS for Injection
- Remove the carton of PAPZIMEOS from the freezer when ready for administration.
- Take the vial out of the pouch and immediately thaw in a 37°C [98.6°F] water bath or dry bead bath with the vial in an upright position until there are no visible ice crystals in the vial. Exposure of the thawed vial to the 37°C [98.6°F] water bath or dry bead bath should be less than or equal to 5 minutes.
- Immediately after thawing, wipe the vial with 70% isopropyl alcohol. Flip off the cap and wipe the rubber stopper top.
- Swirl gently and visually inspect the vial of PAPZIMEOS. PAPZIMEOS should appear as a slightly opalescent to opalescent, colorless liquid, and free of visible particulates. DO NOT use if particulates or discoloration are visible in the suspension.
- Aseptically withdraw 1 mL of PAPZIMEOS from the thawed vial using a 3 mL syringe with an 18G to 22G needle. DO NOT use a filter needle.
- Replace the 18G to 22G needle with a subcutaneous injection needle (23G to 25G).
- Dispose of the used needle and empty vial in a biohazard container.
DO NOT hold PAPZIMEOS at room temperature for more than 60 minutes after thawing.
DO NOT store thawed PAPZIMEOS vials or filled syringes in a refrigerator, freezer, or on dry ice.
Treat any PAPZIMEOS spills with a virucidal agent (such as sodium hypochlorite with 0.5% active chlorine or 6% hydrogen peroxide) for 15 minutes. Dispose of any unused product or waste materials as per facility biohazard waste disposal procedure.
2.3 Administration
Administer PAPZIMEOS via subcutaneous injection with the following procedures:
- Select the lateral regions of the upper arm and thigh for injection. Avoid areas of edema, potential infection, wounds, scars, or the site of a recent subcutaneous injection.
- Clean the injection site thoroughly with an alcohol swab and allow at least 30 seconds to dry.
- Inject PAPZIMEOS by inserting the needle at an angle to ensure delivery in the subcutaneous space.
- Clean the area with an alcohol swab again, DO NOT massage the site of injection.
- Place potentially contaminated materials from the injection site, including dressings, that may have the patient's bodily fluids/waste, in a sealable bag and dispose into regular trash. These precautions should be followed for 1-2 weeks after injection. Practice proper hand hygiene, such as hand washing, when coming into direct contact with patient body waste.
- Avoid direct contact with the injection site (e.g., touching or scratching) and dressings for approximately 24 hours following treatment.
3. Dosage Forms and Strengths
PAPZIMEOS is supplied as a slightly opalescent to opalescent, colorless suspension for subcutaneous injection with a concentration of 5×1011 PU/mL. Each single-dose vial delivers a minimum extractable volume of 1 mL [see How Supplied/Storage and Handling (16)].
5. Warnings and Precautions
5.1 Injection-Site Reactions
Injection-site reactions have occurred with PAPZIMEOS injection. Monitor patients for local site reactions for at least 30 minutes after the initial treatment and manage accordingly.
5.2 Thrombotic Events
Thrombotic events may occur following administration of adenoviral vector-based therapies including PAPZIMEOS due to the potential to induce prothrombotic antibody development. Monitor patients for signs and symptoms of thrombotic events and treat events according to clinical practice.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflects exposure to PAPZIMEOS in one clinical study (Study PRGN-2012-201). A total of 38 adults with recurrent respiratory papillomatosis received a PAPZIMEOS dose of either 1×1011 PU (n=3), or 5×1011 PU (n=35) per injection on Days 1, 15, 43, and 85 [see Clinical Studies (14)]. The most common adverse reactions (incidence ≥5%) are summarized in Table 2.
|
*Graded per NCI CTCAE v5.0. There were no Grade >2 adverse reactions. |
|
| Preferred Term | Grade 1-2*
n (%) |
| Injection site reaction | 37 (97) |
| Fatigue | 28 (74) |
| Chills | 25 (66) |
| Pyrexia | 24 (63) |
| Myalgia | 11 (29) |
| Nausea | 10 (26) |
| Headache | 4 (11) |
| Tachycardia | 3 (8) |
| Diarrhea | 2 (5) |
| Vomiting | 2 (5) |
| Hyperhidrosis | 2 (5) |
Other clinically significant adverse reactions occurring in <5% of patients included vision blurred (3%), injection site bruising (3%), dizziness (3%), dyspnea (3%), and pruritus (3%).
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data with PAPZIMEOS in pregnant women. Animal reproductive and developmental toxicity studies have not been conducted with PAPZIMEOS. In the PRGN-2012-201 study, one patient reported pregnancy at 6 months following completion of treatment with PAPZIMEOS. The patient delivered at 40 weeks without any reported birth complications or neonatal concerns.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information available on the presence of PAPZIMEOS in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PAPZIMEOS and any potential adverse effects on the breastfed child from PAPZIMEOS or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of PAPZIMEOS have not been established in pediatric patients.
8.5 Geriatric Use
There were 9 patients (24%) 65 years of age and older and 1 patient (3%) 75 years of age and older in Study PRGN-2012-201. Clinical studies of PAPZIMEOS did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger patients.
11. Papzimeos Description
PAPZIMEOS (zopapogene imadenovec-drba) is a non-replicating adenoviral vector-based immunotherapy designed to express a fusion antigen comprising selected regions of human papillomavirus (HPV) types 6 and 11 proteins.
PAPZIMEOS has a concentration of 5×1011 PU/mL. Each single-dose vial contains a minimum extractable volume of 1 mL and the following excipients: Tris base (10 mM), sodium chloride (75 mM), magnesium chloride hexahydrate (1 mM), polysorbate 80 (0.019 mM), and trehalose dihydrate (146 mM).
PAPZIMEOS is a sterile, slightly opalescent to opalescent colorless suspension.
The product contains no preservatives.
12. Papzimeos - Clinical Pharmacology
12.1 Mechanism of Action
PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy designed to express a fusion antigen of selected regions of human papillomavirus (HPV) proteins expressed in HPV 6- and HPV 11-infected cells. PAPZIMEOS is designed to generate an immune response directed against HPV 6 and HPV 11 proteins in patients with recurrent respiratory papillomatosis.
12.2 Pharmacodynamics
The pharmacodynamic effect of PAPZIMEOS was evaluated in Study PRGN-2012-201 [see Clinical Studies (14)]. In 28 patients evaluated at completion of treatment, the induction of HPV 6- and HPV 11-specific T cell responses in RRP patients, was higher in RRP patients demonstrating a clinical response to treatment, i.e. reduction in or elimination of the requirement for surgical debulking during the 12 months following completion of treatment, with mean fold-change from baseline of 164.9 versus 5.1 (p<0.018). This difference persisted at 12 weeks post-treatment, with mean fold-change of 61.5 in responders versus 11.5 in non-responders.
14. Clinical Studies
The efficacy of PAPZIMEOS was evaluated in an open-label, single-arm study in adults with recurrent respiratory papillomatosis (PRGN-2012-201; NCT04724980). The study enrolled adults who had histological and clinically diagnosed recurrent respiratory papillomatosis and had 3 or more debulking procedures to remove laryngotracheal papillomas in the 12 months prior to treatment with PAPZIMEOS.
A total of 38 patients received subcutaneous injections of PAPZIMEOS on days 1, 15, 43, and 85. Prior to initiation of treatment with PAPZIMEOS (Day 1), patients underwent a standard-of-care surgical debulking procedure to remove laryngotracheal papillomas. Physicians also had the option to remove any visible papillomas during the treatment interval. Of the 38 patients, 3 patients were treated with PAPZIMEOS at a dose of 1×1011 particle units (PU) per injection. Thirty-five patients were treated at a dose of 5×1011 PU per injection and were included in the efficacy evaluation.
The demographic characteristics of the population were as follows: the median age was 50 years (range 20 to 88 years), 15 patients (39%) were female, 33 patients (87%) were White, 1 patient (3%) was Asian, 1 patient (3%) was African American, 1 patient (3%) was of “other” race, 2 patients (5%) were of unknown race, and 32 patients (84%) were non-Hispanic or Latino. The mean (SD) BMI was 28 (6) kg/m2. The median number of baseline surgical procedures performed in the 12 months prior to treatment was 4 (range 3 to 10). This included the protocol mandated debulking surgery on Day 1 to establish minimal residual disease.
The primary efficacy endpoint was the percentage of patients with a complete response to PAPZIMEOS treatment, defined as no requirement for surgical intervention in the 12 months after treatment.
At a dose of 5×1011 PU per injection, 18 out of 35 patients achieved a complete response at 12 months resulting in a complete response rate of 51% [95% confidence interval (CI) 34 to 69%]. Of the 18 patients with a complete response in the ongoing study, 15 demonstrated continued complete response at 24 months yielding a complete response rate of 43% (95% CI 26 to 61%) at 2 years for the 35 patients in the efficacy population.
At a dose of 1×1011 PU per injection, no patient (0 out of 3) achieved a complete response.
16. How is Papzimeos supplied
16.1 How Supplied
Each carton of PAPZIMEOS (NDC 84768-511-01) contains one single-dose vial (NDC 84768-511-99) of PAPZIMEOS sterile frozen suspension.
PAPZIMEOS is supplied in a single-dose vial made from cyclic olefin polymer (COP) with a rubber stopper and aluminum flip-cap seal. Each vial is sealed inside a pouch (NDC 84768-511-00). The pouch is placed in the container along with a Package Insert. Each vial is formulated to contain an extractable dose of 5×1011 PU in a 1 mL suspension.
16.2 Storage and Handling
PAPZIMEOS is shipped and stored frozen at ≤ -60°C [≤ -76°F] and should be stored in an appropriate freezer at ≤ -60°C [≤ -76°F] until ready to thaw and administer.
DO NOT place the vial in a refrigerator, freezer, or on dry ice at any time once removed from the pouch. Protect the vials from light. DO NOT shake the vial.
PAPZIMEOS should not be held at room temperature for more than 60 minutes after thawing.
PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy. Follow universal biohazard precautions for handling [see Dosage and Administration (2.2)(2.3)] and for the disposal of all vials and syringes.
17. Patient Counseling Information
Discuss following with the patients.
- Injection Site Reactions: Inform patients injection site reactions have occurred after PAPZIMEOS injection. Signs and Symptoms may include reactions such as redness, pain, swelling, itching, or warmth at the injection site. Advise patients to manage symptoms with cold compresses, over the counter pain relievers or antihistamines, if needed. Seek medical care if symptoms worsen or are accompanied by signs of a systemic allergic reaction (difficulty breathing, widespread rash, facial swelling) or infection [see Warnings and Precautions (5.1)].
- Thrombotic Events: Inform patients that thrombotic events may occur after PAPZIMEOS injection. Signs and Symptoms may include shortness of breath, chest pain, leg swelling, persistent abdominal pain, or neurological symptoms (including severe or persistent headaches or blurred vision). Monitor patients for signs and symptoms of thrombotic events and treat events according to clinical practice [see Warnings and Precautions (5.2)].
Manufactured by:
Precigen, Inc.
20358 Seneca Meadows Parkway
Germantown, MD 20876 USA
U.S. License No. 2364
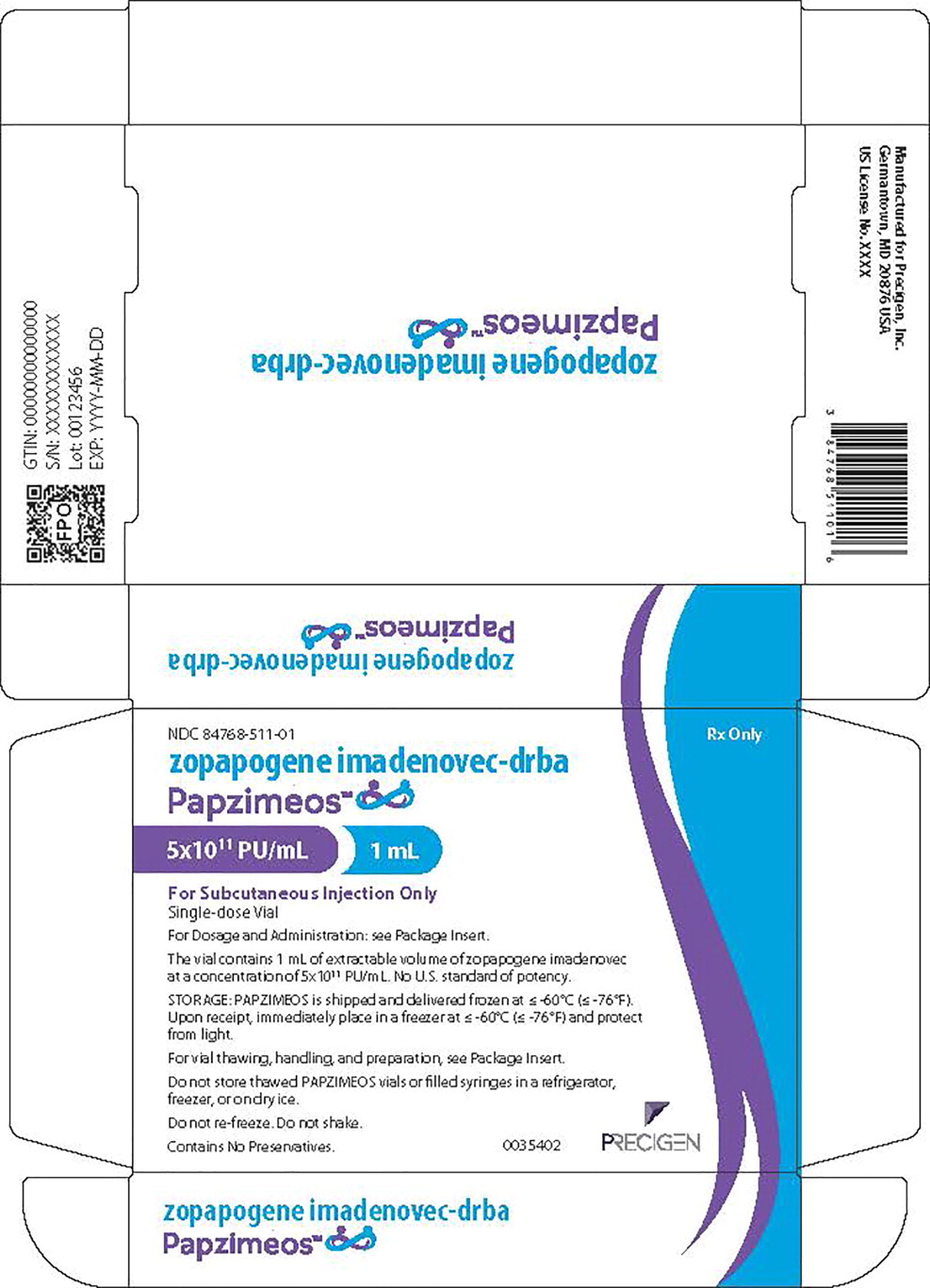
Principal Display Panel – 1 mL Carton Label
NDC 84768-511-01
Rx Only
zopapogene imadenovec-drba
Papzimeos™
5x1011 PU/mL 1 mL
For Subcutaneous Injection Only
Single-dose Vial
For Dosage and Administration: See Package Insert.
The vial contains 1 mL of extractable volume of zopapogene imadenovec
at a concentration of 5x1011 PU/mL. No U.S. standard of potency.
STORAGE: PAPZIMEOS is shipped and delivered frozen at ≤-60°C (≤-76°F).
Upon receipt, immediately place in a freezer at ≤-60°C (≤-76°F) and protect
from light.
For vial thawing, handling, and preparation, see Package Insert.
Do not store thawed PAPZIMEOS vials or filled syringes in a refrigerator,
freezer, or on dry ice.
Do not re-freeze. Do not shake.
Contains No Preservatives.
0035402
PRECIGEN
| PAPZIMEOS
zopapogene imadenovec injection, suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Precigen, Inc. (054652865) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioReliance Corporation | 119271065 | ANALYSIS(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Maryland | 116950534 | MANUFACTURE(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Pharma Solutions, LLC | 014904112 | PACK(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Charles River Laboratories, Inc | 078495006 | ANALYSIS(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nelson Laboratories, LLC | 151663234 | ANALYSIS(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Precigen, Inc. | 054652865 | API MANUFACTURE(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SAFC Carlsbad, Inc. | 053629163 | MANUFACTURE(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SGS Canada, Inc | 203668041 | ANALYSIS(84768-511) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| WuXi Advanced Therapies Inc. | 117556312 | ANALYSIS(84768-511) | |