NuDermRxPAK 120: Package Insert / Prescribing Info
Package insert / product label
Generic name: dimethicone, calcipotriene
Dosage form: cream, kit
On This Page
NuDermRxPAK 120 Description
Calcipotriene Cream, 0.005% contains calcipotriene monohydrate, a synthetic vitamin D 3 derivative, for topical dermatological use.
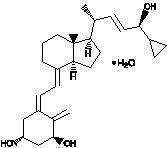
Chemically, calcipotriene monohydrate is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-1α,3β,24-triol monohydrate, with the empirical formula C 27H 40O 3•H 2O, a molecular weight of 430.6, and the following structural formula:
Calcipotriene monohydrate is a white or off-white crystalline substance. Calcipotriene Cream contains calcipotriene monohydrate equivalent to 50 μg/g anhydrous calcipotriene in a cream base of cetearyl alcohol, ceteth-20, diazolidinyl urea, dichlorobenzyl alcohol, dibasic sodium phosphate, edetate disodium, dl-alpha tocopherol, glycerin, mineral oil, petrolatum, and water.
NuDermRxPAK 120 - Clinical Pharmacology
In humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D 3 (cholecalciferol) in the skin. Calcipotriene is a synthetic analog of vitamin D 3.
Clinical studies with radiolabelled calcipotriene ointment indicate that approximately 6% (± 3%, SD) of the applied dose of calcipotriene is absorbed systemically when the ointment is applied topically to psoriasis plaques, or 5% (± 2.6%, SD) when applied to normal skin, and much of the absorbed active is converted to inactive metabolites within 24 hours of application. Systemic absorption of the cream has not been studied.
Vitamin D and its metabolites are transported in the blood, bound to specific plasma proteins. The active form of the vitamin, 1,25-dihydroxy vitamin D 3 (calcitriol), is known to be recycled via the liver and excreted in the bile. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone.
Clinical Studies
Adequate and well-controlled trials of patients treated with Calcipotriene Cream have demonstrated improvement usually beginning after 2 weeks of therapy. This improvement continued with approximately 50% of patients showing at least marked improvement in the signs and symptoms of psoriasis after 8 weeks of therapy, but only approximately 4% showed complete clearing.
Indications and Usage for NuDermRxPAK 120
Calcipotriene Cream, 0.005%, is indicated for the treatment of plaque psoriasis. The safety and effectiveness of topical calcipotriene in dermatoses other than psoriasis have not been established.
Contraindications
Calcipotriene Cream is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. It should not be used by patients with demonstrated hypercalcemia or evidence of vitamin D toxicity. Calcipotriene Cream should not be used on the face.
Warnings
Contact dermatitis, including allergic contact dermatitis, has been observed with the use of Calcipotriene Cream.
Precautions
General
Use of Calcipotriene Cream may cause transient irritation of both lesions and surrounding uninvolved skin. If irritation develops, Calcipotriene Cream should be discontinued.
For external use only. Keep out of the reach of children. Always wash hands thoroughly after use.
Reversible elevation of serum calcium has occurred with use of topical calcipotriene. If elevation in serum calcium outside the normal range should occur, discontinue treatment until normal calcium levels are restored.
Information for Patients
Patients using Calcipotriene Cream should receive the following information and instructions:
- This medication is to be used only as directed by the physician. It is for external use only. Avoid contact with the face or eyes. As with any topical medication, patients should wash their hands after application.
- This medication should not be used for any disorder other than that for which it was prescribed.
- Patients should report to their physician any signs of adverse reactions.
- Patients that apply Calcipotriene Cream to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.).
Carcinogenesis, Mutagenesis, Impairment of Fertility
When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10 and 30 µg/kg/day (corresponding to 9, 30 and 90 µg/m 2/day), no significant changes in tumor incidence were observed when compared to control. In a study in which albino hairless mice were exposed to both UVR and topically applied calcipotriene, a reduction in the time required for UVR to induce the formation of skin tumors was observed (statistically significant in males only), suggesting that calcipotriene may enhance the effect of UVR to induce skin tumors. Patients that apply Calcipotriene Cream to exposed portions of the body should avoid excessive exposure to either natural or artificial sunlight (including tanning booths, sun lamps, etc.). Physicians may wish to limit or avoid use of phototherapy in patients that use Calcipotriene Cream.
Calcipotriene did not elicit any mutagenic effects in an Ames mutagenicity assay, a mouse lymphoma TK locus assay, a human lymphocyte chromosome aberration assay, or in a micronucleus assay conducted in mice.
Studies in rats at doses up to 54 μg/kg/day (324 μg/m 2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance.
Pregnancy
Teratogenic Effects
Studies of teratogenicity were done by the oral route where bioavailability is expected to be approximately 40-60% of the administered dose. Increased rabbit maternal and fetal toxicity was noted at 12 μg/kg/day (132 μg/m 2/day). Rabbits administered 36 μg/kg/day (396 μg/m 2/day) resulted in fetuses with a significant increase in the incidences of pubic bones, forelimb phalanges, and incomplete bone ossification. In a rat study, oral doses of 54 μg/kg/day (318 μg/m 2/day) resulted in a significantly higher incidence of skeletal abnormalities consisting primarily of enlarged fontanelles and extra ribs. The enlarged fontanelles are most likely due to calcipotriene's effect upon calcium metabolism. The maternal and fetal calculated no-effect exposures in the rat (43.2 μg/m 2/day) and rabbit (17.6 μg/m 2/day) studies are approximately equal to the expected human systemic exposure level (18.5 μg/m 2/day) from dermal application. There are no adequate and well-controlled studies in pregnant women. Therefore, Calcipotriene Cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
There is evidence that maternal 1,25-dihydroxy vitamin D 3 (calcitriol) may enter the fetal circulation, but it is not known whether it is excreted in human milk. The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin. Because many drugs are excreted in human milk, caution should be exercised when Calcipotriene Cream is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of Calcipotriene Cream in pediatric patients have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at greater risk than adults of systemic adverse effects when they are treated with topical medication.
Geriatric Use
Of the total number of patients in clinical studies of calcipotriene cream, approximately 15% were 65 or older, while approximately 3% were 75 and over. There were no significant differences in adverse events for subjects over 65 years compared to those under 65 years of age. However, the greater sensitivity of older individuals cannot be ruled out.
Clinical Trials Experience
In controlled clinical trials, the most frequent adverse experiences reported for Calcipotriene Cream, 0.005% were cases of skin irritation, which occurred in approximately 10-15% of patients. Rash, pruritus, dermatitis and worsening of psoriasis were reported in 1 to 10% of patients.
Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions associated with the use of Calcipotriene Cream have been identified post-approval: contact dermatitis including allergic contact dermatitis.
Overdosage
Topically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with excessive use of topical calcipotriene. If elevation in serum calcium should occur, discontinue treatment until normal calcium levels are restored. (See PRECAUTIONS.)
NuDermRxPAK 120 Dosage and Administration
Apply a thin layer of Calcipotriene Cream to the affected skin twice daily and rub in gently and completely. The safety and efficacy of Calcipotriene Cream have been demonstrated in patients treated for eight weeks.
How is NuDermRxPAK 120 supplied
Calcipotriene Cream, 0.005% is available in:
120 gram aluminum tubes (NDC 66993-877-78)

Manufactured for: Prasco Laboratories, Mason, OH 45040 USA
Manufactured by: LEO Laboratories Ltd., Dublin 12, Ireland
To report SUSPECTED ADVERSE REACTIONS, contact LEO Pharma
Inc. at 1-877-494-4536 or FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch
Revised 06/2017
Indications and Usage for NuDermRxPAK 120
Uses
For the treatment and / or prevention of diaper rash temporarily protects and helps relieve chapped or cracked skin
Warnings
For external use only.
- When using this product
- do not get into eyes
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
STOP USE SECTION
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
KEEP OUT OF REACH OF CHILDREN
If swallowed , get medical help or contact a Poison Control Center right away.
NuDermRxPAK 120 Dosage and Administration
Directions
- Cleanse skin with Thera Moisturizing Body Cleanser or Thera Foaming Body Cleanser
- Apply cream liberally until entire area is covered
- Apply as needed
INACTIVE INGREDIENTS
Inactive Ingredients:
Purified Water, Glyceryl Stearate, PEG 100 Stearate, Stearic Acid, Aleurites Moluccana Seed Oil,
Cetyl Alcohol, Glycerin, Butylene Glycol, Carthamus Tinctorius (Safflower) Seed Oil,
Dimethicone Crosspolymer, Calcium Pantothenate (Vitamin B5), Maltodextrin, Niacinamide (Vitamin B3),
Pyridoxine HCL (Vitamin B6), Silica, Sodium Ascorbyl Phosphate (Vitamin C), Sodium Starch Octenylsuccinate,
Tocopheryl Acetate (Vitamin E), Disodium EDTA, Sodium Hyaluronate, Aloe Barbadensis Leaf Extract,
Zingiber Officinale (Ginger) Root Extract, Carthamus Tinctorius (Safflower) Oleosomes, Fragrance,
Phenoxyethanol, Caprylyl Glycol, Chlorphenesin, Bisabolol, Lysine, Histidine, Arginine, Aspartic Acid,
Threonine, Serine, Glutamic Acid, Proline, Glycine, Alanine, Valine, Methionine, Isoleucine, Leucine,
Tyrosine, Phenylalanine, Cysteine, Triethanolamine
| NUDERMRXPAK 120
calcipotriene, dimethicone kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - NuCare Pharmaceuticals,Inc. (010632300) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NuCare Pharmaceuticals,Inc. | 010632300 | manufacture(70859-056) | |