MenQuadfi: Package Insert / Prescribing Info
Package insert / product label
Generic name: Meningococcal (Groups A, C, Y, W) Conjugate Vaccine
Dosage form: solution for intramuscular injection
Medically reviewed by Drugs.com. Last updated on Jun 2, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
MenQuadfi, Meningococcal (Groups A, C, Y, W) Conjugate Vaccine
injection, for intramuscular use
Initial U.S. Approval: 2020
Recent Major Changes
Indications and Usage for MenQuadfi
MenQuadfi is a vaccine indicated for active immunization for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, W, and Y. MenQuadfi is approved for use in individuals 6 weeks of age and older. (1)
MenQuadfi does not prevent N. meningitidis serogroup B disease. (1)
MenQuadfi Dosage and Administration
0.5 mL dose for intramuscular use. (2)
Primary Vaccination
- Infants aged from 6 weeks: 4-dose series administered at 2, 4, 6, and 12 through 18 months of age. The first dose may be given as early as 6 weeks of age. (2.2)
- Infants aged 6 months through 11 months: 2-dose series with the second dose administered in the second year of life and at least 3 months after the first dose. (2.2)
- Infants aged 12 months through 23 months: 2-dose series with the second dose administered at least 3 months after the first dose. (2.2)
- Individuals 2 years of age and older: A single dose. (2.2)
Booster Vaccination
- A single dose of MenQuadfi may be administered to individuals 13 years of age and older who are at continued risk for meningococcal disease if at least 3 years have elapsed since a prior dose of meningococcal (groups A, C, W, Y) conjugate vaccine. (2.2)
Vaccination Following Prior Dose of Meningococcal Polysaccharide Vaccine
- A single dose of MenQuadfi may be administered if at least 3 years have elapsed since a prior dose of meningococcal polysaccharide vaccine. (2.2)
Dosage Forms and Strengths
MenQuadfi is an injection. A single dose is 0.5 mL. (3)
Contraindications
Severe allergic reaction to any component of the vaccine, or after a previous dose of MenQuadfi or any other tetanus toxoid-containing vaccine. (4)
Adverse Reactions/Side Effects
Most commonly reported adverse reactions (≥10%) were as follows:
- Infants 6 weeks through 23 months of age:
- 4-dose series given at 2, 4, 6, and 12 through 18 months of age: tenderness (38.5%–45.6%), erythema (12.5%–19.5%), and swelling (9.6%–12.7%) at the injection site; irritability (40.1%–51.9%), crying abnormal (27.3%–42.1%), drowsiness (25.1%–43.4%), appetite lost (17.3%–21.8%), fever (7.8%–17.6%), vomiting (3.5%–13.2%). (6)
- 2-dose series given at 6 through 7 months and 12 through 13 months of age: tenderness (30.1%–42.7%), erythema (21.1%–21.8%), and swelling (14.5%–16.0%) at the injection site; irritability (40.0%–49.0%), crying abnormal (26.6%–35.0%), drowsiness (27.7%–36.5%), appetite lost (15.2%–17.1%), fever (9.3%–12.9%), vomiting (5.5%–8.5%). (6)
- Children 2 through 9 years of age: pain (38.6%), erythema (22.6%), and swelling (13.8%) at the injection site; malaise (21.1%), myalgia (20.1%), and headache (12.5%). (6)
- Adolescents aged 10 through 17 years of age: injection site pain (34.8%–45.2%), myalgia (27.4%–35.3%), headache (26.5%–30.2%), and malaise (19.4%–26.0%). (6)
- Adults aged 18 through 55 years: injection site pain (41.9%), myalgia (35.6%), headache (29.0%), and malaise (22.9%). (6)
- Adults 56 years of age and older: pain at the injection site (25.5%), myalgia (21.9%), headache (19.0%), and malaise (14.5%). (6)
In adolescents and adults, rates of solicited adverse reactions following a booster dose were comparable to those observed following primary vaccination. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2025
Full Prescribing Information
1. Indications and Usage for MenQuadfi
MenQuadfi® is a vaccine indicated for active immunization for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, W, and Y. MenQuadfi is approved for use in individuals 6 weeks of age and older.
MenQuadfi does not prevent N. meningitidis serogroup B disease.
2. MenQuadfi Dosage and Administration
2.1 Preparation for Administration
MenQuadfi is a clear, colorless solution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If any of these conditions exist, the vaccine should not be administered.
2.2 Dose and Schedule
Administer MenQuadfi as a single 0.5 mL injection intramuscularly.
Primary Vaccination:
| Age at First Dose | Primary Vaccination Schedule |
|---|---|
| 2 months | 4-dose series at 2, 4, 6, and 12 through 18 months of age. The first dose may be given as early as 6 weeks. |
| 6 months through 11 months | 2-dose series with the second dose administered in the second year of life and at least 3 months after the first dose. |
| 12 months through 23 months | 2-dose series with the second dose administered at least 3 months after the first dose. |
| 2 years and older | A single dose |
4. Contraindications
Severe allergic reaction to any component of the vaccine, or after a previous dose of MenQuadfi or any other tetanus toxoid-containing vaccine [see Description (11)].
5. Warnings and Precautions
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of MenQuadfi.
5.2 Altered Immunocompetence
Reduced Immune Response
Some individuals with altered immunocompetence, including some individuals receiving immunosuppressant therapy, may have reduced immune responses to MenQuadfi.
Complement Deficiency
Persons with certain complement deficiencies and persons receiving treatment that inhibits terminal complement activation (for example, eculizumab) are at increased risk for invasive disease caused by N. meningitidis, including invasive disease caused by serogroups A, C, W, and Y, even if they develop antibodies following vaccination with MenQuadfi [see Clinical Pharmacology (12.1)].
5.3 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, including MenQuadfi. Procedures should be in place to avoid injury from fainting.
5.4 Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) has been reported in temporal relationship following administration of another U.S.-licensed meningococcal quadrivalent polysaccharide conjugate vaccine. The decision by the healthcare professional to administer MenQuadfi to persons with a history of GBS should take into account the expected benefits and potential risks.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial(s) of a vaccine cannot be directly compared to rates in the clinical trial(s) of another vaccine and may not reflect the rates observed in practice.
The safety of MenQuadfi in individuals 6 weeks of age and older was evaluated in ten randomized, active-controlled, multi-center clinical studies conducted in the U.S. and Puerto Rico. A total of 4273 participants younger than 2 years of age received at least one dose of either a 4-dose series (N=3807) or a 2-dose series (N=466) of MenQuadfi and were included in the safety analyses. In studies that enrolled participants 2 years of age and older, a total of 5787 participants received a single dose, either as a primary dose (N=4517), a booster dose (N=1119) of MenQuadfi following priming with a meningococcal conjugate vaccine, or a dose of MenQuadfi following a prior dose of meningococcal polysaccharide vaccine (N=151) and were included in the safety analyses.
Safety Monitoring
Participants were monitored for immediate reactions for 30 minutes following each vaccination while at the study site. Solicited injection site and systemic reactions were recorded by participants or by parents/guardians in a diary card at home daily for 7 days following vaccination. At subsequent visits approximately 1 to 2 months post-vaccination, participants or parents/guardians reviewed diary cards with study investigators and when necessary, made corrections or clarifications. All unsolicited adverse events that occurred within 30 days following vaccination were recorded by participants or by parents/guardians, then collected and reported by the investigator. Unsolicited adverse events that were medically attended (i.e., visits to an emergency room, or an unexpected visit to a health care provider), and all serious adverse events (SAEs) were collected for at least 6 months after vaccination for all studies except Study 10 [NCT04142242], in which these safety data were collected for at least 1 month.
Primary Vaccination
Infants 6 weeks through 23 months of age
The safety of MenQuadfi in infants aged 6 weeks through 23 months of age who received at least one dose and up to 4 doses of MenQuadfi was evaluated in three clinical studies.
4-Dose Series (infants initiating vaccination at 2 months of age):
The safety of MenQuadfi in infants from 6 weeks of age vaccinated with a 4-dose series (at 2, 4, 6, and 12 through 18 months) was evaluated in Study 1 (NCT03537508). The safety analysis set included 1727 participants who received at least one dose of MenQuadfi (1375 participants received a full 4-dose series) and 867 participants received at least one dose of Menveo® [Meningococcal (Groups A, C, Y, and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine] (705 participants received a full 4-dose series). Each of these vaccines was administered concomitantly with U.S. licensed vaccines including Pentacel® (diphtheria, tetanus, acellular pertussis, inactivated polio and Haemophilus influenzae type b vaccine [DTaP-IPV/Hib]) given at 2, 4, 6, and at 15 through 18 months of age; Prevnar 13® (pneumococcal 13-valent conjugate vaccine [PCV13]) given at 2, 4, 6, and 12 months of age; RotaTeq® (rotavirus vaccine) given at 2, 4, and 6 months of age; Engerix-B® (hepatitis B vaccine [HepB]) given at 2 and 6 months of age (first dose given 28 days prior to study); M-M-R II® (measles, mumps, and rubella vaccine [MMR]) and Varivax® (varicella vaccine) given at 12 months of age; and Havrix® (hepatitis A vaccine [HepA]) given at 15 through 18 months of age. Of the participants who received MenQuadfi (N=1727), 47.2% were female, 81.9% were White, 11.8% were Black or African American, 0.8% were Asian, 4.4% were of other racial groups, and 48.1% were of Hispanic or Latino ethnicity. There were no substantive differences in demographic characteristics between vaccine groups.
Among participants in Study 1 who received at least one dose of MenQuadfi, 133 (7.7%) were born preterm (31 to < 37 weeks of gestational age); of these 133 preterm infants, 121 were born 34 to < 37 weeks of gestation. Among participants who received at least one dose of Menveo, 58 (6.7%) were born preterm; of these 58 preterm infants, 47 were born 34 to < 37 weeks of gestation. There were no notable differences in the rates and severity of adverse reactions between preterm and full-term infants after any vaccination.
The rates and severity of the solicited adverse reactions that occurred within 7 days following each dose of MenQuadfi and comparator vaccines are presented in Table 2.
| Dose 1 | Dose 2 | Dose 3 | Dose 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MenQuadfi N†=1552–1625 % | Menveo N†=789–824 % | MenQuadfi N†=1456–1519 % | Menveo N†=743–786 % | MenQuadfi N†=1391–1458 % | Menveo N†=701–748 % | MenQuadfi (12 through 15 months)‡ N†=798–864 % | Menveo (12 months) N†=419–438 % | MenQuadfi (15 through 18 months)‡ N†=383–401 % | Pentacel and Havrix without MenACWY (15 through 18 months) N†=184–199 % |
||
| Pentacel | Havrix | ||||||||||
|
|||||||||||
| Local Reactions | |||||||||||
| Injection Site Tenderness§ | 45.6 | 43.3 | 43.6 | 43.9 | 42.4 | 40.6 | 38.5 | 40.6 | 40.8 | 39.7 | 37.7 |
| Grade 3 | 4.4 | 3.4 | 3.4 | 3.3 | 3.2 | 2.4 | 2.2 | 2.3 | 1.8 | 3.0 | 2.0 |
| Injection Site Erythema¶ | 12.5 | 11.4 | 18.3 | 16.3 | 19.5 | 19.7 | 16.2 | 20.6 | 18.7 | 16.1 | 12.1 |
| Grade 3 | 0 | 0.1 | 0 | 0.3 | 0 | 0.1 | 0.1 | 0 | 0.5 | 1.0 | 0.5 |
| Injection Site Swelling¶ | 9.6 | 9.0 | 12.3 | 10.1 | 12.7 | 13.0 | 10.2 | 15.1 | 12.3 | 14.6 | 11.1 |
| Grade 3 | < 0.1 | 0 | < 0.1 | 0.3 | 0 | 0.1 | 0.1 | 0 | 0 | 1.0 | 0.5 |
| Systemic Reactions | |||||||||||
| Fever# | 7.8 | 6.6 | 17.6 | 17.9 | 15.2 | 16.5 | 14.8 | 14.3 | 11.0 | 8.2 | |
| Grade 3 | < 0.1 | 0 | 0.6 | 0.8 | 0.7 | 0.3 | 0.5 | 1.2 | 1.0 | 0.5 | |
| VomitingÞ | 13.2 | 10.9 | 9.7 | 8.5 | 9.5 | 6.7 | 4.6 | 5.7 | 3.5 | 2.0 | |
| Grade 3 | 0.5 | 0.2 | 0.3 | 0.5 | 0.3 | 0.5 | 0.1 | 0.2 | 0.3 | 0 | |
| Crying abnormalß | 40.9 | 39.4 | 42.1 | 41.5 | 37.6 | 36.6 | 32.1 | 35.2 | 27.3 | 27.3 | |
| Grade 3 | 2.8 | 2.3 | 3.6 | 2.8 | 3.0 | 1.7 | 2.9 | 2.5 | 1.3 | 2.0 | |
| Drowsinessà | 43.4 | 42.3 | 38.2 | 38.8 | 35.3 | 34.5 | 33.6 | 34.2 | 25.1 | 25.3 | |
| Grade 3 | 2.5 | 2.7 | 1.9 | 2.4 | 2.0 | 2.1 | 2.2 | 1.1 | 1.0 | 2.0 | |
| Appetite lostè | 20.5 | 20.4 | 19.9 | 22.9 | 18.9 | 19.0 | 21.8 | 21.5 | 17.3 | 17.7 | |
| Grade 3 | 0.9 | 0.7 | 0.6 | 0.9 | 1.0 | 0.8 | 0.9 | 2.1 | 0.8 | 1.5 | |
| Irritabilityð | 51.9 | 51.0 | 51.4 | 50.9 | 47.4 | 46.3 | 46.9 | 47.0 | 40.1 | 38.9 | |
| Grade 3 | 3.9 | 2.8 | 4.7 | 5.2 | 4.3 | 2.7 | 4.2 | 3.0 | 2.0 | 4.0 | |
The safety of MenQuadfi in infants from 6 weeks of age vaccinated with a 4-dose series (at 2, 4, 6, and 12 months) was evaluated in Study 2 (NCT03673462). The safety analysis set included 2080 participants who received at least one dose of MenQuadfi (1836 participants received a full 4-dose series) and 697 participants received at least one dose of Menveo (622 participants received a full 4-dose series). Each of these vaccines was administered concomitantly with U.S. licensed vaccines including Pentacel (DTaP-IPV/Hib) given at 2, 4, and 6 months of age; Prevnar 13 (PCV13) given at 2, 4, 6, and 12 months of age; RotaTeq (rotavirus vaccine) given at 2, 4, and 6 months of age; Engerix-B (HepB) given at 2 and 6 months of age (first dose given 28 days prior to study); M-M-R II (MMR) and Varivax (varicella vaccine) given at 12 months of age. Of the participants who received MenQuadfi (N=2080), 47.7% were female, 81.9% were White, 10.1% were Black or African American, 1.3% were Asian, 5.7% were of other racial groups, and 26.8% were of Hispanic or Latino ethnicity. There were no substantive differences in demographic characteristics between vaccine groups.
Among participants in Study 2 who received at least one dose of MenQuadfi, 89 (4.3%) were born preterm (31 to < 37 weeks of gestational age); of these 89 preterm infants, 83 were born 34 to < 37 weeks of gestation. Among participants who received at least one dose of Menveo, 20 (2.9%) were born preterm at 34 to < 37 weeks of gestation. There were no notable differences in the rates and severity of adverse reactions between preterm and full-term infants after any vaccination.
In Study 1 and Study 2, following any dose in the 4-dose series, SAEs were reported by 5.3% of participants following MenQuadfi and 3.6% of participants following Menveo during the entire study period (which includes the first study vaccination through 6 months after the final vaccination). SAEs were reported by 0.6% of participants following MenQuadfi and 0.3% of participants reported SAEs following Menveo within 7 days after vaccination. SAEs were reported by 2.2% of participants following MenQuadfi and 1.2% of participants following Menveo within 30 days after vaccination.
There were two SAEs of febrile seizure considered possibly related to vaccination with MenQuadfi following the fourth dose when concomitantly administered with MMR, varicella, and PCV13 vaccines at 12 months of age (Study 2). One case occurred one day post vaccination. The other case occurred 9 days post vaccination. Neither febrile seizure was reported as prolonged, and neither study participant reported a recurrent febrile seizure through 6 months after the last dose of MenQuadfi.
2-Dose Series (infants initiating vaccination at 6 months through 23 months of age):
The safety of MenQuadfi in infants from 6 months of age vaccinated with a 2-dose series (at 6 through 7 months and 12 through 13 months) was evaluated in Study 3 (NCT03691610). The safety analysis set included 370 participants who received at least one dose of MenQuadfi (309 participants received a full 2-dose series) and 361 participants who received at least one dose of Menveo (302 participants received a full 2-dose series). Each of these vaccines was administered concomitantly with U.S. licensed vaccines including Pentacel (DTaP-IPV/Hib) or Pediarix® (DTaP-IPV-HepB), ActHIB® or Hiberix® or PedvaxHIB® (Hib vaccine), RotaTeq (rotavirus vaccine), and Engerix-B (HepB) given at 6 months of age; Prevnar 13 (PCV13) given at 6 and 13 months of age; and M-M-R II (MMR) and Varivax (varicella vaccine) given at 12 months of age. Of the participants who received MenQuadfi (N=370), 47.6% were female, 74.1% were White, 17% were Black or African American, 1.6% were Asian, 7.3% were of other racial groups, and 45.1% were of Hispanic or Latino ethnicity. There were no substantive differences in demographic characteristics between the vaccine groups.
The rates and severity of the solicited adverse reactions that occurred within 7 days following MenQuadfi compared with Menveo (Study 3) are presented in Table 3.
| Dose 1 | Dose 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| MenQuadfi N†=341–351 % | Menveo N†=325–339 % | MenQuadfi N†=279–290 % | Menveo N†=261–273 % |
|||||
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 |
|
||||||||
| Local Reactions | ||||||||
| Injection Site Tenderness‡ | 42.7 | 2.3 | 34.7 | 2.1 | 30.1 | 0.7 | 32.0 | 1.5 |
| Injection Site Erythema§ | 21.1 | 0.0 | 21.6 | 0.0 | 21.8 | 0.0 | 21.7 | 0.7 |
| Injection Site Swelling§ | 16.0 | 0.0 | 15.7 | 0.0 | 14.5 | 0.3 | 14.7 | 0.4 |
| Systemic Reactions | ||||||||
| Fever¶ | 12.9 | 0.3 | 12.3 | 0.6 | 9.3 | 0.7 | 7.7 | 0.8 |
| Vomiting# | 8.5 | 0.3 | 8.0 | 0.0 | 5.5 | 0.3 | 3.7 | 0.4 |
| Crying abnormalÞ | 35.0 | 1.7 | 32.4 | 1.2 | 26.6 | 0.7 | 25.3 | 0.7 |
| Drowsinessß | 36.5 | 2.3 | 38.9 | 2.4 | 27.7 | 0.7 | 31.5 | 1.1 |
| Appetite lostà | 17.1 | 1.4 | 16.2 | 0.6 | 15.2 | 0.7 | 17.3 | 0.7 |
| Irritabilityè | 49.0 | 4.8 | 45.1 | 3.5 | 40.0 | 3.1 | 40.4 | 1.8 |
Following any dose in the 2-dose series administered at 6 through 7 months and 12 through 13 months of age, SAEs were reported by 1.6% of participants following MenQuadfi and 3.3% of participants following Menveo during the entire study period (which includes the first study vaccination through 6 months after the final vaccination). No SAE were reported within 7 days following MenQuadfi and 0.3% of participants reported an SAE following Menveo. SAEs were reported by 0.3% of participants following MenQuadfi and 0.6% of participants following Menveo within 30 days after vaccination. No SAEs were determined to be vaccine related.
In Study 3, safety was also assessed in 199 participants vaccinated with the 2-dose series at 17 through 19 months and 20 through 23 months of age. The safety analysis set included 96 participants who received at least one dose of MenQuadfi (86 participants received a full 2-dose series) and 103 participants who received at least one dose of Menactra® [Meningococcal (Groups A, C, Y, and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine] (96 participants who received a full 2-dose series). Of the participants who received MenQuadfi (N=96), 50% were female, 82.3% were White, 11.5% were Black or African American, 2.1% were Asian, 4.2% were of other racial groups, and 32.3% were of Hispanic or Latino ethnicity. There were no substantive differences in demographic characteristics between the vaccine groups.
The rates and severity of the solicited adverse reactions that occurred within 7 days following MenQuadfi compared with Menactra (Study 3) are presented in Table 4.
| Dose 1 | Dose 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| MenQuadfi N†=89–90 % | Menactra N†=98–99 % | MenQuadfi N†=82 % | Menactra N†=91–93 % |
|||||
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 |
|
||||||||
| Local Reactions | ||||||||
| Injection Site Tenderness‡ | 34.4 | 0.0 | 35.4 | 1.0 | 41.5 | 0.0 | 26.9 | 1.1 |
| Injection Site Erythema§ | 22.2 | 0.0 | 22.2 | 0.0 | 25.6 | 0.0 | 16.1 | 0.0 |
| Injection Site Swelling§ | 18.9 | 0.0 | 12.1 | 0.0 | 20.7 | 0.0 | 7.5 | 0.0 |
| Systemic Reactions | ||||||||
| Fever¶ | 11.2 | 1.1 | 12.2 | 1.0 | 11.0 | 0.0 | 8.8 | 0.0 |
| Vomiting# | 4.4 | 0.0 | 6.1 | 0.0 | 3.7 | 0.0 | 3.2 | 1.1 |
| Crying abnormalÞ | 26.7 | 1.1 | 26.3 | 2.0 | 25.6 | 0.0 | 19.4 | 1.1 |
| Drowsinessß | 24.4 | 0.0 | 23.2 | 2.0 | 23.2 | 0.0 | 14.0 | 1.1 |
| Appetite lostà | 21.1 | 0.0 | 23.2 | 1.0 | 20.7 | 0.0 | 25.8 | 1.1 |
| Irritabilityè | 40.0 | 3.3 | 43.4 | 3.0 | 35.4 | 1.2 | 33.3 | 3.2 |
Following any dose in the 2-dose series administered at 17 through 19 months and 20 through 23 months of age, SAEs were reported by 1.0% of participants following MenQuadfi and 3.9% of participants following Menactra during the entire study period (which includes the first study vaccination through 6 months after the final vaccination). No SAEs were reported within 7 or 30 days following MenQuadfi. SAEs were reported by 1.0% of participants within 7 days and 1.9% of participants within 30 days following Menactra. Among participants who received MenQuadfi, no SAEs were determined to be vaccine related.
No notable differences in the rates and severity of solicited adverse reactions were observed following a 2-dose series when initiated at 6 months or 17 months of age.
Children 2 through 9 years of age
The safety of MenQuadfi in children 2 years through 9 years of age was evaluated in Study 4 (NCT03077438). The safety analysis set included 498 participants who received MenQuadfi and 494 participants who received Menveo. Of the participants 2 through 9 years of age who received MenQuadfi (N=498), 50.2% were 2 through 5 years of age, 49.8% were 6 through 9 years of age, 49.0% were female, 80.5% were White, 13.3% were Black or African American, 0.4% were Asian, 5.2% were of other racial groups, and 22.9% were of Hispanic or Latino ethnicity. There were no substantive differences in demographic characteristics between the vaccine groups.
The rates and severity of the solicited adverse reactions that occurred within 7 days following MenQuadfi compared with Menveo (Study 4) are presented in Table 5.
SAEs were reported at a rate of 1.4% following MenQuadfi and at a rate of 0.6% following Menveo during the entire study period. Most SAEs were reported more than 30 days following vaccination and were commonly occurring events in the general population in this age group. No SAEs were determined to be vaccine related.
| MenQuadfi (N†=484–487) % | Menveo (N†=479–486) % |
|||
|---|---|---|---|---|
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 |
| Local Reactions | ||||
| Injection Site Pain‡ | 38.6 | 0.6 | 42.4 | 1.0 |
| Injection Site Erythema§ | 22.6 | 3.1 | 31.5 | 9.9 |
| Injection Site Swelling§ | 13.8 | 1.4 | 21.5 | 5.6 |
| Systemic Reactions | ||||
| Myalgia¶ | 20.1 | 0.4 | 23.0 | 0.8 |
| Malaise¶ | 21.1 | 1.8 | 20.4 | 1.0 |
| Headache¶ | 12.5 | 0.0 | 11.5 | 0.4 |
| Fever# | 1.9 | 0.0 | 2.7 | 0.4 |
Adolescents 10 through 17 years of age
The safety of MenQuadfi in adolescents 10 through 17 years of age was evaluated in two clinical trial studies Study 5 (NCT02199691) and Study 6 (NCT02842853). The safety analysis set in these two studies included 3,196 participants who received MenQuadfi alone (1,684 participants), MenQuadfi concomitantly with Adacel® [Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed] (Tdap) and Gardasil® [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant] (HPV) (392 participants), the concomitant vaccines without MenQuadfi (296 participants), or a U.S.-licensed comparator meningococcal vaccine (824 participants). The comparator meningococcal vaccine was either Menveo (501 participants) or Menactra (323 participants).
Of the participants 10 through 17 years of age who received MenQuadfi (N=1684), 49.6% were female. Among those with reported race and ethnicity, 79.3% were White, 14.2% were Black or African American, 1.1% were Asian, 5.4% were of other racial groups, and 21.5% were of Hispanic or Latino ethnicity. Mean age was 11.9 years at time of administration. There were no substantive differences in demographic characteristics between the vaccine groups.
The rates and severity of the solicited adverse reactions that occurred within 7 days following MenQuadfi compared with Menveo and Menactra are presented in Table 6. The most common injection site and systemic reactions occurring after MenQuadfi administration (in Study 5 and Study 6) were injection site pain (45.2% and 34.8%) and myalgia (35.3% and 27.4%), respectively.
In Study 5, SAEs were reported by 0.8% of participants following MenQuadfi and 0.8% of participants following Menveo. In Study 6, SAEs were reported by 0.3% of participants following MenQuadfi and 0.9% of participants following Menactra. No SAEs were determined to be vaccine related.
| Study 5 | Study 6 | |||||||
|---|---|---|---|---|---|---|---|---|
| MenQuadfi (N‡=494–496) % | Menveo (N‡=488–491) % | MenQuadfi (N‡=1129–1159) % | Menactra (N‡=310–314) % |
|||||
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 |
| Local Reactions | ||||||||
| Injection Site Pain§ | 45.2 | 1.4 | 42.5 | 1.0 | 34.8 | 1.8 | 41.4 | 2.2 |
| Injection Site Erythema¶ | 5.0 | 0.4 | 7.5 | 1.2 | 4.5 | 0.3 | 4.5 | 0.3 |
| Injection Site Swelling¶ | 5.4 | 0.2 | 6.5 | 0.4 | 4.1 | < 0.1 | 4.8 | 0.0 |
| Systemic Reactions | ||||||||
| Myalgia§ | 35.3 | 1.6 | 35.2 | 1.8 | 27.4 | 1.9 | 31.2 | 1.9 |
| Headache§ | 30.2 | 1.8 | 30.9 | 1.8 | 26.5 | 2.3 | 28.0 | 1.9 |
| Malaise§ | 26.0 | 2.2 | 26.4 | 2.8 | 19.4 | 1.2 | 23.9 | 1.3 |
| Fever# | 1.4 | 0.4 | 1.2 | 0.6 | 0.7 | 0.2 | 0.6 | 0.0 |
Among 296 participants who received Tdap and HPV concomitantly (without MenQuadfi) and 392 participants who received MenQuadfi concomitantly with Tdap and HPV, there were no notable differences in the rates of systemic solicited adverse reactions within 7 days following vaccination.
Dizziness within 30 minutes following vaccination was experienced by 1 (0.2%) participant who received MenQuadfi in Study 5 (NCT02199691) and 2 (0.2%) participants who received MenQuadfi in Study 6 (NCT02842853). Three participants in Study 5 experienced syncope within 30 minutes following vaccination: 1 (0.2%) participant who received Menveo, 1 (0.3%) participant who received MenQuadfi concomitantly with Tdap and HPV, and 1 (0.3%) participant who received Tdap and HPV concomitantly (without MenQuadfi). These events were non-serious and spontaneously resolved on the same day.
Adults 18 through 55 years of age
The safety of MenQuadfi in adults 18 through 55 years of age was evaluated in Study 6 (NCT02842853). The safety analysis set included 1495 participants who received MenQuadfi and 312 participants who received Menactra. Of the participants 18 years through 55 years of age who received MenQuadfi (N=1495), 65.2% were female. Among those with reported race and ethnicity, 73.3% were White, 21.0% were Black or African American, 2.2% were Asian, 3.5% were of other racial groups, and 20.0% were of Hispanic or Latino ethnicity. Mean age was 39.4 years at time of administration.
The rates and severity of the solicited adverse reactions that occurred within 7 days following MenQuadfi compared with Menactra are presented in Table 7.
Dizziness within 30 minutes following vaccination was experienced by 5 (0.3%) participants who received MenQuadfi and 1 (0.3%) participant who received Menactra. These events were non-serious and spontaneously resolved on the same day.
SAEs were reported by 1.6% of participants following MenQuadfi and 0.6% of participants following Menactra during the entire study period. No SAEs were determined to be vaccine related.
| MenQuadfi (N†=1441–1460) % | Menactra (N†=297–301) % |
|||
|---|---|---|---|---|
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 |
| Local Reactions | ||||
| Injection Site Pain‡ | 41.9 | 1.9 | 35.0 | 1.3 |
| Injection Site Erythema§ | 5.1 | 0.3 | 3.7 | 0.3 |
| Injection Site Swelling§ | 4.3 | 0.2 | 3.4 | 0.3 |
| Systemic Reactions | ||||
| Myalgia‡ | 35.6 | 3.6 | 31.2 | 2.3 |
| Headache‡ | 29.0 | 2.9 | 27.6 | 2.7 |
| Malaise‡ | 22.9 | 2.9 | 18.9 | 3.3 |
| Fever¶ | 1.4 | 0.1 | 1.7 | 0.7 |
Adults 56 years of age and older
The safety of MenQuadfi in adults 56 years of age and older was evaluated in Study 7 (NCT02842866). The safety analysis set included 448 participants who received MenQuadfi intramuscularly and 453 participants who received a non-conjugate comparator meningococcal vaccine, Menomune® – A/C/Y/W-135 [Meningococcal Polysaccharide Vaccine, Groups A, C, Y, and W-135 Combined], subcutaneously. Of the participants 56 years of age and older who received MenQuadfi (N=448), 44.4% were 56 through 64 years of age, 55.6% were 65 years of age and older, 57.6% were female, 86.6% were White, 11.6% were Black or African American, 1.1% were Asian, 0.4% were of other racial groups and 7.8% were of Hispanic or Latino ethnicity. Mean age was 67.0 years at time of administration.
The rates and severity of the solicited adverse reactions that occurred within 7 days following MenQuadfi compared with Menomune in Study 7 (NCT02842866) are presented in Table 8.
SAEs were reported by 3.3% of participants following MenQuadfi and 3.3% of participants following Menomune during the entire study period. No SAEs were determined to be vaccine related.
| MenQuadfi (N†=436–443) % | Menomune‡ (N†=449–451) % |
|||
|---|---|---|---|---|
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 |
| Local Reactions | ||||
| Injection Site Pain§ | 25.5 | 0.7 | 9.6 | 0.7 |
| Injection Site Erythema¶ | 5.2 | 0.2 | 0.0 | 0.0 |
| Injection Site Swelling¶ | 4.5 | 0.0 | 0.0 | 0.0 |
| Systemic Reactions | ||||
| Myalgia§ | 21.9 | 1.6 | 15.3 | 1.3 |
| Headache§ | 19.0 | 0.7 | 14.6 | 0.7 |
| Malaise§ | 14.5 | 1.4 | 11.3 | 1.8 |
| Fever# | 2.1 | 0.2 | 0.4 | 0.0 |
Booster Vaccination Following Priming with a Meningococcal Conjugate Vaccine; Vaccination Following a Prior Dose of a Meningococcal Polysaccharide Vaccine
Adolescents and adults 15 years of age and older
The safety of MenQuadfi in previously vaccinated adolescents and adults 15 years of age and older was evaluated in Study 8 (NCT02752906). All randomized participants had received a primary dose of either (Menveo or Menactra) 4 to 10 years previously. The safety analysis set included 402 participants who received a single booster dose of MenQuadfi (median age: 17.8 years) and 407 participants who received a single booster dose of Menactra (median age: 17.9 years). Of the participants who received MenQuadfi, 51.5% were female, 85.1% were White, 9.7% were Black, 2.7% were Asian and 2.2% were of other racial groups, and 15.7% were of Hispanic or Latino ethnicity.
The most commonly reported solicited adverse reactions (≥10%) within 7 days of MenQuadfi booster vaccination were injection site pain (44.7%) and headache (37.9%), myalgia (36.7%), and malaise (27.6%). The majority of solicited adverse reactions were Grade 1 or 2 and resolved within 3 days. Compared with recipients of a Menactra booster dose, recipients of a MenQuadfi booster dose had higher rates of injection site erythema (MenQuadfi 5.0%, Menactra 1.5%) and swelling (MenQuadfi 4.0%, Menactra 0.7%). Overall rates of solicited adverse reactions were comparable to those observed in unvaccinated adolescents and adults after a single MenQuadfi dose.
SAEs were reported by 1.2% of participants following MenQuadfi and 1.0% of participants following Menactra during the entire study period. No SAEs were determined to be vaccine related.
Adolescents and adults 13 through 26 years of age
The safety of MenQuadfi in previously vaccinated adolescents and adults 13 through 26 years of age was evaluated in Study 9 (NCT04084769). All randomized participants had received a primary dose of either MenQuadfi or Menveo 3–6 years previously. The safety analysis set included 370 participants who received a booster dose of MenQuadfi alone (median age: 15.0 years for participants primed with MenQuadfi and 16.0 years for participants primed with Menveo) and 185 participants who received MenQuadfi concomitantly with Trumenba® [Meningococcal Group B Vaccine] (N=93, median age: 15.0 years) or Bexsero® [Meningococcal Group B Vaccine] (N=92, median age: 15.0 years). Of the participants who received a booster dose of MenQuadfi, 47.2% were female, 88.1% were White, 8.2% were Black, 3.8% were of other racial groups, and 14.4% were of Hispanic or Latino ethnicity.
The rates and severity of the solicited adverse reactions that occurred within 7 days following a booster dose of MenQuadfi alone or concomitantly with Trumenba or Bexsero in Study 9 (NCT04084769) are presented in Table 9.
The majority of solicited reactions were Grade 1 or 2 and resolved within 3 days after vaccination.
There were no reported SAEs that were assessed as vaccine related.
| MenQuadfi in MenQuadfi-primed (N=186) % | MenQuadfi in Menveo-primed (N=184) % | MenQuadfi and Trumenba in MenQuadfi-primed (N=93) % | MenQuadfi and Bexsero in MenQuadfi-primed (N=92) % |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 |
| N: number of participants in the safety analysis set | ||||||||
| Local Reactions† | ||||||||
| Injection Site Pain | 38.2 | 0.5 | 33.7 | 1.1 | 48.9 | 5.4 | 56.5 | 0 |
| Injection Site Erythema | 6.5 | 0.5 | 5.4 | 0 | 1.1 | 0 | 6.5 | 1.1 |
| Injection Site Swelling | 5.4 | 0 | 1.6 | 0 | 2.2 | 0 | 5.4 | 1.1 |
| Systemic Reactions | ||||||||
| Myalgia | 32.8 | 1.6 | 34.8 | 1.1 | 65.2 | 7.6 | 63.0 | 4.3 |
| Headache | 36.0 | 1.1 | 34.8 | 1.6 | 42.4 | 4.3 | 39.1 | 2.2 |
| Malaise | 26.9 | 2.2 | 25.5 | 2.2 | 39.1 | 5.4 | 40.2 | 3.3 |
| Fever | 0 | 0 | 2.2 | 0.5 | 1.1 | 0 | 4.4 | 0 |
Older adults ≥ 59 years of age
The safety of MenQuadfi in previously vaccinated older adults ≥ 59 years of age was evaluated in Study 10 (NCT04142242). All randomized participants had received a prior dose of either MenQuadfi (N=162) or Menomune (N=151) at a median interval of 3.34 and 3.35 years, respectively. The safety analysis set included 313 participants who received a dose of MenQuadfi (median age: 69.0 years for participants primed with MenQuadfi and 70.0 years for participants who received a prior dose of Menomune); 62.6% were female, 90.4% were White, 8.6% were Black, 0.3% were of other racial groups, and 10.5% were of Hispanic or Latino ethnicity.
The rates and severity of the solicited adverse reactions that occurred within 7 days following a dose of MenQuadfi in Study 10 (NCT04142242) are presented in Table 10.
The majority of solicited reactions were Grade 1 or 2 and resolved within 3 days after vaccination.
There were no reported SAEs that were assessed as vaccine related.
| MenQuadfi-primed (N=162) % | Prior dose of Menomune (N=151) % |
|||
|---|---|---|---|---|
| Adverse Reactions | Any | Grade 3 | Any | Grade 3 |
| N: number of participants in the safety analysis set | ||||
|
||||
| Local Reactions | ||||
| Injection Site Pain | 16.7 | 0 | 21.2 | 0.7 |
| Injection Site Erythema | 3.7 | 0 | 7.3 | 0 |
| Injection Site Swelling | 3.7 | 0 | 4.6 | 0 |
| Systemic Reactions | ||||
| Myalgia | 21.6 | 2.5 | 19.9 | 1.3 |
| Headache | 18.5 | 0 | 13.9 | 0 |
| Malaise | 13.6 | 1.9 | 14.6 | 2.6 |
| Fever | 0.6 | 0 | 0 | 0 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of MenQuadfi. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
Immune system disorder: Anaphylaxis and other forms of hypersensitivity.
Nervous system disorder: Febrile seizure, convulsion, syncope.
7. Drug Interactions
7.1 Concomitant Administration with Other Vaccines
In a clinical trial in adolescents 10 through 17 years of age, MenQuadfi was administered concomitantly with Tdap and HPV [see Adverse Reactions (6) and Clinical Studies (14.3)].
Lower geometric mean antibody concentrations (GMCs) for antibodies to the pertussis antigens filamentous hemagglutinin (FHA), pertactin (PRN) and fimbriae (FIM) were observed when MenQuadfi was co-administered with Tdap and HPV, compared to concomitant administration of Tdap and HPV (without MenQuadfi) [see Clinical Studies (14.3)].
7.2 Immunosuppressive Treatments
Immunosuppressive therapies may reduce the immune response to MenQuadfi [see Warnings and Precautions (5)].
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to MenQuadfi during pregnancy. To enroll in or obtain information about the registry, call Sanofi Pasteur at 1-800-822-2463.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
There are no clinical studies of MenQuadfi in pregnant women. Available human data on MenQuadfi administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study in female rabbits administered a full human dose (0.5 mL) prior to mating and during gestation period revealed no evidence of harm to the fetus due to MenQuadfi (see Animal Data).
Data
Animal Data
In a developmental toxicity study, female rabbits received a human dose of MenQuadfi by intramuscular injection on five occasions: 30 days and 10 days prior to mating, gestation days 6, 12 and 27. No adverse effects on pre-weaning development up to post-natal day 35 were observed. There were no vaccine-related fetal malformations or variations observed.
8.2 Lactation
Risk Summary
It is not known whether MenQuadfi is excreted in human milk. Data are not available to assess the effects of MenQuadfi on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for MenQuadfi and any potential adverse effects on the breastfed child from MenQuadfi or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness of MenQuadfi have not been established in individuals younger than 6 weeks of age.
Safety and effectiveness of MenQuadfi have been established in individuals from 6 weeks through 17 years of age [see Adverse Reactions (6) and Clinical Studies (14)].
8.5 Geriatric Use
A total of 249 participants 65 years of age and older, including 71 participants 75 years of age or older, in Study 7 received one dose of MenQuadfi [see Adverse Reactions (6.1) and Clinical Studies (14.1)].
MenQuadfi recipients ≥ 65 years of age had lower GMTs and seroresponse rates for all serogroups compared to MenQuadfi recipients 56 through 64 years of age [see Clinical Studies (14.1)].
11. MenQuadfi Description
MenQuadfi [Meningococcal (Groups A, C, Y, W) Conjugate Vaccine] is a sterile injection for intramuscular use that contains Neisseria meningitidis serogroup A, C, W, and Y capsular polysaccharide antigens that are individually conjugated to tetanus toxoid protein. N. meningitidis A, C, W, and Y strains are cultured on Mueller Hinton agar medium and grown in Watson Scherp medium. The polysaccharides are extracted from the N. meningitidis cells and purified by centrifugation, detergent precipitation, alcohol precipitation, solvent extraction, and diafiltration. To prepare the polysaccharides for conjugation, Serogroup A is activated with carbonyldiimidazole (CDI), derivatized with adipic acid dihydrazide (ADH), and purified by diafiltration. Serogroups C, W, and Y are depolymerized, activated with periodate, and purified by diafiltration.
Clostridium tetani is fermented in media to generate tetanus toxin, which is purified by ammonium sulfate precipitation to yield purified tetanus toxin (PTT) and detoxified with formaldehyde to yield purified tetanus protein (PTP). The PTP is then concentrated and filtered to yield concentrated tetanus protein (CTP). The activated/derivatized polysaccharides are covalently linked to tetanus toxoid and purified by chromatography and serial diafiltration. The four meningococcal components, present as individual serogroup-specific glycoconjugates, compose the final formulated vaccine.
MenQuadfi is manufactured as a sterile, clear, colorless solution. Each 0.5 mL dose of vaccine contains 10 microgram each of meningococcal A, C, W, and Y polysaccharide antigens conjugated to approximately 55 micrograms tetanus toxoid protein carrier; 3.35 mg sodium chloride (0.67%), and 1.23 mg sodium acetate (30 mM). Potency of MenQuadfi is determined by quantifying the amount of each polysaccharide antigen that is conjugated to tetanus toxoid protein and the amount of unconjugated polysaccharide present.
MenQuadfi does not contain a preservative. Each 0.5 mL dose may contain residual amounts of formaldehyde of less than 3 mcg/mL, by calculation.
The vial in which the vaccine components are contained is composed of USP Type I borosilicate glass. The vial stopper is a chlorobutyl synthetic polyisoprene blend stopper (not made with natural rubber latex).
12. MenQuadfi - Clinical Pharmacology
12.1 Mechanism of Action
Invasive meningococcal disease (IMD) is caused by the bacterium N. meningitidis, a gram-negative diplococcus found exclusively in humans. The presence of bactericidal anti-capsular meningococcal antibodies in serum has been associated with protection from IMD. MenQuadfi induces the production of bactericidal antibodies specific to the capsular polysaccharides of N. meningitidis serogroups A, C, W, and Y.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
MenQuadfi has not been evaluated for carcinogenic or mutagenic potential or for impairment of male fertility. MenQuadfi administered to female rabbits had no effects on fertility [see Use in Specific Populations (8.1)].
14. Clinical Studies
To infer effectiveness of MenQuadfi, the immunogenicity in persons 6 weeks of age and older was evaluated using a serogroup-specific serum bactericidal assay with exogenous human complement (hSBA). Serum was collected at baseline and 30 days post-vaccination to measure antibodies with hSBA. The hSBA geometric mean titers (GMTs) and proportion of participants who achieved hSBA seroresponse (defined below) were evaluated.
In all studies, the seroresponse rate for each serogroup was defined as: the proportion of participants with an hSBA
- pre-vaccination titer < 1:8 who achieved a post-vaccination titer ≥ 1:16, or
- pre-vaccination titer ≥ 1:8 who achieved a post-vaccination titer at least 4-fold greater than the pre-vaccination titer.
14.1 Primary Vaccination
Immunogenicity of a 4-dose series administered at 2, 4, 6, and 12 through 18 months of age
Study 1 evaluated the effectiveness of MenQuadfi, based on immune responses measured by hSBA, in comparison to Menveo following a 4-dose series (given at 2, 4, 6, and 12 through 15 months) when concomitantly administered with U.S. licensed vaccines [see Adverse Reactions (6.1)].
Immune non-inferiority was based on 1) percentage of participants with hSBA titers ≥ 1:8 following the 3rd dose of the 4-dose series and 2) seroresponse rates following completion of a 4-dose series at 2, 4, 6, and 12 through 15 months of age. Non-inferiority was met for both co-primary endpoints [the lower limit (LL) of the 2-sided 95% CI of the difference in rates (MenQuadfi – Menveo) was > -10%] for all four serogroups.
The percentage of participants with hSBA titers ≥ 1:8, hSBA seroresponse rate, and GMTs are presented in Table 11.
| Post 3rd Dose† | Post 4th Dose‡ | |||||
|---|---|---|---|---|---|---|
| Endpoint | MenQuadfi (95% CI) | Menveo (95% CI) | Percent difference MenQuadfi minus Menveo (95% CI) | MenQuadfi (95% CI) | Menveo (95% CI) | Percent difference MenQuadfi minus Menveo (95% CI) |
| N: number of participants in per-protocol analysis set with valid serology results. 95% CI of the single proportion calculated from the exact binomial method |
||||||
|
||||||
| A | N=682–852 | N=322–409 | N=501–642 | N=223–296 | ||
| % ≥1:8 | 77.9 (75.0; 80.7) | 67.7 (63.0; 72.2) | 10.21§ (4.98; 15.59) | 87.7 (84.9; 90.1) | 88.2 (83.9; 91.6) | |
| % Participants achieving Seroresponse¶ | 64.4 (60.6; 68.0) | 50.6 (45.0; 56.2) | 79.4 (75.6; 82.9) | 77.6 (71.5; 82.9) | 1.86# (-4.38; 8.64) | |
| GMT | 25 (23; 28) | 15 (13; 18) | 67 (58; 78) | 57 (47; 70) | ||
| C | N=691–835 | N=338–421 | N=530–655 | N=238–300 | ||
| % ≥1:8 | 99.0 (98.1; 99.6) | 91.2 (88.1; 93.7) | 7.83§ (5.31; 10.96) | 99.4 (98.4; 99.8) | 93.3 (89.9; 95.9) | |
| % Participants achieving Seroresponse¶ | 96.4 (94.7; 97.6) | 82.8 (78.4; 86.7) | 97.0 (95.1; 98.3) | 88.2 (83.4; 92.0) | 8.75# (4.80; 13.60) | |
| GMT | 391 (356; 428) | 53 (46; 61) | 678 (606; 758) | 91 (76; 109) | ||
| W | N=739–883 | N=369–438 | N=540–651 | N=250–305 | ||
| % ≥1:8 | 98.6 (97.6; 99.3) | 92.9 (90.1; 95.1) | 5.72§ (3.44; 8.57) | 99.4 (98.4; 99.8) | 99.0 (97.2; 99.8) | |
| % Participants achieving Seroresponse¶ | 92.8 (90.7; 94.6) | 85.6 (81.6; 89.1) | 97.6 (95.9; 98.7) | 96.4 (93.3; 98.3) | 1.19# (-1.18; 4.45) | |
| GMT | 98 (91; 106) | 49 (43; 55) | 387 (352; 426) | 175 (149; 206) | ||
| Y | N=701–861 | N=347–423 | N=523–651 | N=233–295 | ||
| % ≥1:8 | 98.3 (97.1; 99.0) | 91.7 (88.7; 94.2) | 6.53§ (4.01; 9.62) | 99.1 (98.0; 99.7) | 98.6 (96.6; 99.6) | |
| % Participants achieving Seroresponse¶ | 88.7 (86.2; 91.0) | 81.8 (77.4; 85.8) | 96.4 (94.4; 97.8) | 92.3 (88.1; 95.4) | 4.09# (0.68; 8.44) | |
| GMT | 88 (81; 96) | 41 (36; 46) | 296 (268; 327) | 186 (158; 219) | ||
The percentage of participants with hSBA titers ≥ 1:8 prior to the 4th dose of MenQuadfi (given at 12 through 15 months of age, N=607–619) or Menveo (given at 12 months of age, N=282–288), respectively, was 62.8% and 46.5% for serogroup A, 93.1% and 30.6% for serogroup C, 97.1% and 61.5% for serogroup W, and 96.2% and 67.9% for serogroup Y. GMTs prior to the 4th dose of MenQuadfi or Menveo, respectively, were 11 and 7 for serogroup A, 61 and 4 for serogroup C, 58 and 9 for serogroup W, and 44 and 10 for serogroup Y.
In descriptive analyses, there were no notable differences in hSBA vaccine seroresponse rates observed with the administration of the 4th dose of MenQuadfi at 12 through 15 months of age or 15 through 18 months of age.
Immunogenicity of a 2-dose series administered between 6 and 23 months of age
Immunogenicity of MenQuadfi compared to Menveo following a 2-dose series with the first dose administered at 6 through 7 months and the second dose administered at 12 through 13 months when concomitantly administered with U.S. licensed vaccines was evaluated in Study 3 [see Adverse Reactions (6.1)].
Immune non-inferiority was demonstrated based on the percentage of participants with hSBA titers ≥ 1:8 and hSBA seroresponse rates following administration of 2 doses [the lower limit of the 2-sided 95% CI of the difference in rates (MenQuadfi – Menveo) was > -10%] for all four serogroups.
The percentage of participants with hSBA titers ≥ 1:8, hSBA seroresponse rate, and GMTs are presented in Table 12.
| Post 2nd Dose | |||
|---|---|---|---|
| Endpoint | MenQuadfi (95% CI) | Menveo (95% CI) | Percent difference MenQuadfi minus Menveo (95% CI) |
| N: number of participants in per-protocol analysis set with valid serology results. 95% CI of the single proportion calculated from the exact binomial method |
|||
|
|||
| A | N=141–170 | N=123–158 | |
| % ≥1:8 | 95.3 (90.9; 97.9) | 93.0 (87.9; 96.5) | 2.26† (-3.01; 7.83) |
| % Participants achieving Seroresponse‡ | 89.4 (83.1; 93.9) | 82.9 (75.1; 89.1) | 6.43§ (-1.92; 15.08) |
| GMT | 184 (143; 237) | 119 (90.6; 157) | |
| C | N=134–162 | N=126–160 | |
| % ≥1:8 | 100 (97.7; 100) | 98.1 (94.6; 99.6) | 1.88† (-0.75; 5.37) |
| % Participants achieving Seroresponse‡ | 99.3 (95.9; 100) | 97.6 (93.2; 99.5) | 1.63§ (-2.07; 6.06) |
| GMT | 1473 (1236; 1756) | 319 (263; 388) | |
| W | N=143–171 | N=127–159 | |
| % ≥1:8 | 100 (97.9; 100) | 95.6 (91.1; 98.2) | 4.40† (1.25; 8.81) |
| % Participants achieving Seroresponse‡ | 99.3 (96.2; 100) | 92.9 (86.9; 96.7) | 6.39§ (1.81; 12.25) |
| GMT | 442 (367; 533) | 106 (83; 135) | |
| Y | N=140–170 | N=128–160 | |
| % ≥1:8 | 100 (97.9; 100) | 97.5 (93.7; 99.3) | 2.50† (-0.18; 6.25) |
| % Participants achieving Seroresponse‡ | 98.6 (94.9; 99.8) | 97.7 (93.3; 99.5) | 0.92§ (-3.03; 5.36) |
| GMT | 423 (358; 499) | 133 (107; 166) | |
The percentage of participants with hSBA titers ≥ 1:8 prior to the 2nd dose of MenQuadfi (N=103–106) or Menveo (N=91–94), respectively, was 77.7% and 73.6% for serogroup A, 98.1% and 69.1% for serogroup C, 96.2% and 50.5% for serogroup W, and 96.2% and 54.8% for serogroup Y. GMTs prior to the 2nd dose of MenQuadfi or Menveo, respectively, were 20 and 15 for serogroup A, 150 and 13 for serogroup C, 47 and 6 for serogroup W, and 46 and 7 for serogroup Y.
The immunogenicity of MenQuadfi compared to Menactra following a 2-dose series with the first dose administered at 17 through 19 months and the second dose at 20 through 23 months of age was also descriptively evaluated in Study 3. The seroresponse rates following the second dose of MenQuadfi (N=59–61) or Menactra (N=58–65), respectively, at 20 through 23 months of age were 72.9% and 46.6% for serogroup A, 100% and 93.2% for serogroup C, 100% and 76.3% for serogroup W, and 100% and 88.1% for serogroup Y. The percentage of participants with hSBA titers ≥ 1:8 following the second dose of MenQuadfi or Menactra, respectively, were 88.5% and 62.5% for serogroup A, 100% and 98.5% for serogroup C, 100% and 84.6% for serogroup W, and 100% and 92.3% for serogroup Y. hSBA GMTs following the second dose of MenQuadfi or Menactra, respectively, were 45 and 13 for serogroup A, 1727 and 59 for serogroup C, 202 and 25 for serogroup W, and 284 and 46 for serogroup Y.
Immunogenicity in Children 2 through 9 Years of Age
Immunogenicity of MenQuadfi compared to Menveo in participants 2 through 9 years of age was evaluated in Study 4 (NCT03077438). The hSBA seroresponse rate and GMTs are presented in Table 13.
Immune non-inferiority, based on seroresponse rates, was demonstrated for MenQuadfi as compared to Menveo for all four serogroups.
| Endpoint† | MenQuadfi (95% CI) | Menveo (95% CI) | Percent difference MenQuadfi minus Menveo‡
(95% CI) |
|---|---|---|---|
| N: number of participants in per-protocol analysis set with valid serology results. 95% CI of the single proportion calculated from the exact binomial method. 95% CI of the difference calculated from the Wilson Score method without continuity correction. |
|||
|
|||
| A | N=455–456 | N=458 | |
| % Participants achieving Seroresponse | 55.4 (50.7; 60.0) | 47.8 (43.2; 52.5) | 7.6 (1.1, 14.0) |
| GMT | 25 (22; 28) | 23 (20; 26) | |
| C | N=458 | N=458–459 | |
| % Participants achieving Seroresponse | 95.2 (92.8; 97.0) | 47.8 (43.2; 52.5) | 47.4 (42.2, 52.2) |
| GMT | 238 (209; 270) | 17.0 (14; 20) | |
| W | N=458 | N=459 | |
| % Participants achieving Seroresponse | 78.8 (74.8; 82.5) | 64.1 (59.5; 68.4) | 14.8 (8.9, 20.5) |
| GMT | 38 (34; 42) | 26 (23; 30) | |
| Y | N=458 | N=459 | |
| % Participants achieving Seroresponse | 91.5 (88.5; 93.9) | 79.3 (75.3; 82.9) | 12.2 (7.7, 16.7) |
| GMT | 69 (61; 77) | 44 (38; 50) | |
Immunogenicity in Adolescents 10 through 17 Years of Age
Immunogenicity of MenQuadfi compared to Menveo in participants 10 through 17 years of age was evaluated in Study 5 (NCT02199691). Study 5 was conducted in healthy meningococcal vaccine naïve participants and evaluated seroresponse rates following administration with either MenQuadfi alone, Menveo alone, MenQuadfi co-administered with Tdap, and HPV, or Tdap and HPV alone. The hSBA seroresponse rate and GMTs for Study 5 are presented in Table 13.
Immune non-inferiority, based on seroresponse, was demonstrated for MenQuadfi as compared to Menveo for all four serogroups.
Study 5 (NCT02199691) was conducted in healthy meningococcal vaccine naïve male and female participants and evaluated seroresponses following administration with either MenQuadfi alone; Menveo alone; MenQuadfi co-administered with Tdap, and HPV; or Tdap and HPV alone. The hSBA seroresponse rate and GMTs for the MenQuadfi alone and Menveo alone groups are presented in Table 14.
| Endpoint† | MenQuadfi (95% CI) | Menveo (95% CI) | Percent difference MenQuadfi minus Menveo‡ (95% CI) |
|---|---|---|---|
| N: number of participants in per-protocol analysis set with valid serology results. 95% CI of the single proportion calculated from the exact binomial method. 95% CI of the difference calculated from the Wilson Score method without continuity correction. |
|||
|
|||
| A | N=463 | N=464 | |
| % Participants achieving Seroresponse | 70.2 (65.8; 74.3) | 60.3 (55.7; 64.8) | 9.8 (3.7;15.9) |
| GMT | 44 (39; 50) | 35 (30; 41) | |
| C | N=462 | N=463 | |
| % Participants achieving Seroresponse | 96.1 (93.9, 97.7) | 61.6 (57.0, 66.0) | 34.5 (29.7; 39.3) |
| GMT | 387 (329; 456) | 51 (41; 64) | |
| W | N=463 | N=464 | |
| % Participants achieving Seroresponse | 84.2 (80.6; 87.4) | 56.0 (51.4; 60.6) | 28.2 (22.5; 33.7) |
| GMT | 87 (78; 97) | 36 (32; 41) | |
| Y | N=462–463 | N=464 | |
| % Participants achieving Seroresponse | 91.1 (88.2; 93.6) | 66.8 (62.3;71.1) | 24.3 (19.2; 29.3) |
| GMT | 76 (66; 87) | 28 (24; 32) | |
Study 6 evaluated the immunogenicity of MenQuadfi (N=1097–1098) compared to Menactra (N=300) in healthy meningococcal-naïve participants 10 through 17 years of age. Seroresponse rates for MenQuadfi were noninferior to those of Menactra for all serogroups based on the same non-inferiority criteria defined for Study 5.
Immunogenicity in Adults 18 through 55 Years of Age
Immunogenicity of MenQuadfi compared to Menactra in participants 18 through 55 years of age was evaluated in Study 6 (NCT02842853). The hSBA seroresponse rate and GMTs are presented in Table 15.
Immune non-inferiority, based on seroresponse rates, was demonstrated for MenQuadfi as compared to Menactra for all four serogroups.
| Endpoint† | MenQuadfi (95% CI) | Menactra (95% CI) | Percent difference MenQuadfi minus Menactra‡ (95% CI) |
|---|---|---|---|
| N: number of participants in per-protocol analysis set with valid serology results. 95% CI of the single proportion calculated from the exact binomial method. 95% CI of the difference calculated from the Wilson Score method without continuity correction. |
|||
|
|||
| A | N=1406–1408 | N=293 | |
| % Participants achieving Seroresponse | 73.5 (71.2; 75.8) | 53.9 (48.0; 59.7) | 19.6 (13.5; 25.8) |
| GMT | 106 (97; 117) | 52 (43; 64) | |
| C | N=1406–1408 | N=293 | |
| % Participants achieving Seroresponse | 83.4 (81.4; 85.3) | 42.3 (36.6; 48.2) | 41.1 (35.0; 46.9) |
| GMT | 234 (210; 261) | 37 (29; 49) | |
| W | N=1408–1410 | N=293 | |
| % Participants achieving Seroresponse | 77.0 (74.7; 79.2) | 50.2 (44.3; 56.0) | 26.8 (20.7; 32.9) |
| GMT | 76 (69; 83) | 33 (26; 42) | |
| Y | N=1408–1410 | N=293 | |
| % Participants achieving Seroresponse | 88.1 (86.3; 89.8) | 60.8 (54.9; 66.4) | 27.4 (21.7; 33.3) |
| GMT | 219 (200; 239) | 55 (42; 70) | |
Immunogenicity in Adults 56 Years of Age and Older
Immunogenicity of MenQuadfi compared to Menomune in participants 56 years and older was evaluated in Study 7 (NCT02842866).
Enrollment was stratified by age category: 56 through 64 years of age (44.3%), 65 through 74 years of age (39.7%), and 75 years of age and older (15.9%). The overall mean age of participants who received MenQuadfi was 66.9 years; range: 56 through 89.8 years of age. The mean age for participants in the 56 through 64 years age stratum who received MenQuadfi was 60.4 years, the mean age for participants ≥ 65 years age stratum who received MenQuadfi was 72.2 years.
The hSBA seroresponse rate and GMTs are presented in Table 16.
Immune non-inferiority, based on seroresponse rates, was demonstrated for MenQuadfi as compared to Menomune for all four serogroups.
| Endpoint† | MenQuadfi (95% CI) | Menomune (95% CI) | Percent difference MenQuadfi minus Menomune‡ (95% CI) |
|---|---|---|---|
| N: number of participants in per-protocol analysis set with valid serology results. 95% CI of the single proportion calculated from the exact binomial method. 95% CI of the difference calculated from the Wilson Score method without continuity correction. |
|||
|
|||
| A | N=433 | N=431 | |
| % Participants achieving Seroresponse | 58.2 (53.4; 62.9) | 42.5 (37.7; 47.3) | 15.7 (9.08; 22.2) |
| GMT | 55 (47; 65) | 31 (27; 37) | |
| C | N=433 | N=431 | |
| % Participants achieving Seroresponse | 77.1 (72.9; 81.0) | 49.7 (44.8; 54.5) | 27.5 (21.2; 33.5) |
| GMT | 101 (84; 123) | 25 (21; 30) | |
| W | N=433 | N=431 | |
| % Participants achieving Seroresponse | 62.6 (57.8; 67.2) | 44.8 (40.0; 49.6) | 17.8 (11.2; 24.2) |
| GMT | 28 (24; 33) | 15 (13; 18) | |
| Y | N=433 | N=431 | |
| % Participants achieving Seroresponse | 74.4 (70.0; 78.4) | 43.4 (38.7; 48.2) | 31.0 (24.6; 37.0) |
| GMT | 69 (59; 81) | 21 (17; 25) | |
14.2 Booster Vaccination Following Priming with a Meningococcal Conjugate Vaccine; Vaccination Following a Prior Dose of a Meningococcal Polysaccharide Vaccine
Immunogenicity in Adolescents and Adults at least 15 Years of Age and Older
Immunogenicity of a booster dose of MenQuadfi compared to a booster dose of Menactra was evaluated in Study 8 (NCT02752906). The study-enrolled participants 15 years of age and older who had received a primary dose of Menveo or Menactra 4 to 10 years previously.
Immune non-inferiority, based on seroresponse rates, was demonstrated for MenQuadfi as compared to Menactra for all four serogroups.
For a description of study design and number of participants, see section 6.1 Booster Vaccination Following Priming with a Meningococcal Conjugate Vaccine; Vaccination Following a Prior Dose of a Meningococcal Polysaccharide Vaccine. The primary immunogenicity endpoint was hSBA seroresponse to each serogroup 30 days following booster vaccination with MenQuadfi or Menactra given to participants who received a prior dose of Menveo or Menactra 4 to 10 years ago. The other endpoints included the proportions of participants with post-vaccination hSBA ≥1:8 and the hSBA GMTs for each serogroup. These endpoints were also evaluated at 6 days post vaccination in a subset.
Seroresponse rates at Day 30 following booster vaccination with MenQuadfi were 92.2% for serogroup A, 97.1% for serogroup C, 98.2% for serogroup W, and 97.4% for serogroup Y, as compared to 87.1% for serogroup A, 91.8% for serogroup C, 90.7% for serogroup W, and 95.6% for serogroup Y, following booster vaccination with Menactra. At Day 6, following booster vaccination with MenQuadfi, seroresponse rates were 72.7%, 83.6%, 94.5%, and 90.9% for serogroups A, C, W, and Y, respectively.
The hSBA GMTs were 173, 334, 499, and 302 for serogroups A, C, W, and Y at Day 6, and 497, 2618, 1747, and 2070, respectively, for the 4 serogroups at Day 30 following booster dose of MenQuadfi.
Overall, similar seroresponse rates were observed for those participants who received booster vaccination with Menactra.
Immunogenicity in Adolescents and Adults 13 through 26 Years of Age
Immunogenicity of a booster dose of MenQuadfi was evaluated in Study 9 (NCT04084769). The study enrolled participants 13 through 26 years of age who had received a primary dose of MenQuadfi or Menveo 3–6 years previously in Study 5 or Study 6.
For a description of study design and number of participants, see section 6.1 Booster Vaccination Following Priming with a Meningococcal Conjugate Vaccine; Vaccination Following a Prior Dose of a Meningococcal Polysaccharide Vaccine. The primary immunogenicity endpoints were hSBA seroresponse to each serogroup 30 days following a booster vaccination with MenQuadfi given to participants who received a prior dose of MenQuadfi or Menveo 3–6 years previously (Table 17). The other endpoints included hSBA GMTs for each serogroup. These endpoints were also evaluated at 6 days post vaccination in a subset (Per-Protocol Analysis Set 1).
| †Endpoint by Serogroup | MenQuadfi-primed (95% CI) N=174 | Menveo-primed (95% CI) N=176 |
|---|---|---|
| N: number of participants in Per-Protocol Analysis Set 2 (D30) with valid serology results. | ||
|
||
| A | ||
| % Participants achieving Seroresponse | 94.8 (90.4; 97.6) | 93.2 (88.4; 96.4) |
| C | ||
| % Participants achieving Seroresponse | 97.1 (93.4; 99.1) | 98.9 (96.0; 99.9) |
| W | ||
| % Participants achieving Seroresponse | 97.7 (94.2; 99.4) | 98.9 (96.0; 99.9) |
| Y | ||
| % Participants achieving Seroresponse | 98.9 (95.9; 99.9) | 100 (97.9; 100) |
Seroresponse rates at Day 6 following booster dose with MenQuadfi were 82.6%, 89.1%, 97.8%, and 95.7% for serogroups A, C, W, and Y, respectively, in MenQuadfi-primed participants (N=46) and 77.8%, 93.3%, 88.9%, and 91.1% for serogroups A, C, W, and Y, respectively, in Menveo-primed participants (N=45).
Following a booster dose of MenQuadfi, the hSBA GMTs at Day 6 were 289, 3799, 1928, and 1658 for MenQuadfi-primed participants (N=46) and 161, 919, 708, and 800 for Menveo-primed participants (N=45) for serogroups A, C, W, and Y, respectively. At D30, the hSBA GMTs were 502, 3708, 2290, and 2308 for MenQuadfi-primed participants (N=174) and 399, 2533, 2574, and 3036 for Menveo-primed participants (N=176).
Prior to booster vaccination, the percentage of participants with hSBA titers ≥1:8 for serogroups A, C, W, and Y were 71.3%, 87.9%, 86.2%, and 79.9% for those who received a prior dose of MenQuadfi 3–6 years earlier (N=174), and 71.0%, 50.6%, 77.8%, and 52.8% for those who received a prior dose of Menveo 3–6 years earlier (N=176).
Immunogenicity in Older Adults ≥ 59 Years of Age
Immunogenicity of a dose of MenQuadfi was evaluated in Study 10 (NCT04142242). The study enrolled participants ≥ 59 years of age who had received a prior dose of MenQuadfi or Menomune at least 3 years previously in Study 7 (NCT02842866).
For a description of study design and number of participants, see section 6.1 Booster Vaccination Following Priming with a Meningococcal Conjugate Vaccine; Vaccination Following a Prior Dose of a Meningococcal Polysaccharide Vaccine. The primary immunogenicity endpoint was hSBA seroresponse to each serogroup 30 days following vaccination with MenQuadfi in participants who had received a prior dose of Menomune 3 years previously. Additionally, hSBA seroresponse 30 days following vaccination with MenQuadfi in MenQuadfi-primed participants was also assessed (Table 18). The other endpoints included the hSBA GMTs for each serogroup. These endpoints were also evaluated at 6 days post vaccination in a subset (Per-Protocol Analysis Set 2).
| †Endpoint by Serogroup | MenQuadfi-primed (95% CI) | Prior dose of Menomune (95% CI) |
|---|---|---|
| N: number of participants in Per-Protocol Analysis Set 1 (D30) with valid serology results | ||
|
||
| A | N=145 | N=130 |
| % Participants achieving Seroresponse | 79.3 (71.8; 85.6) | 60.8 (51.8; 69.2) |
| C | ||
| % Participants achieving Seroresponse | 93.1 (87.7; 96.6) | 55.0 (46.0; 63.8) |
| W | ||
| % Participants achieving Seroresponse | 90.3 (84.3; 94.6) | 49.2 (40.4; 58.1) |
| Y | ||
| % Participants achieving Seroresponse | 92.4 (86.8; 96.2) | 49.2 (40.4; 58.1) |
Seroresponse rates at Day 6 following vaccination with MenQuadfi were 36.2%, 77.6%, 70.7%, and 72.4% for serogroups A, C, W, and Y, respectively, in MenQuadfi-primed participants (N=58) and 8.1%, 8.1%, 6.5%, and 8.1% for serogroups A, C, W, and Y, respectively, in participants who received a prior dose of Menomune (N=62).
Following vaccination with MenQuadfi, the hSBA GMTs at Day 6 were 44, 206, 118, and 151 for MenQuadfi-primed participants (N=58) and 13, 11, 10, and 11 for participants who received a prior dose of Menomune (N=62) for serogroups A, C, W, and Y, respectively. At D30, the hSBA GMTs were 162, 638, 419, and 566 for MenQuadfi-primed participants (N=145) and 57, 56, 31, and 41 for participants who received a prior dose of Menomune (N=130).
Prior to MenQuadfi vaccination, the percentage of participants with hSBA titers ≥1:8 for serogroups A, C, W, and Y were 64.8%, 73.8%, 66.9%, and 72.4% for those who received a prior dose of MenQuadfi at least 3 years earlier (N=145), and 65.4%, 49.2%, 40.0%, and 41.5% for those who received a prior dose of Menomune at least 3 years earlier (N=130).
14.3 Immunogenicity of Concomitantly Administered Vaccines
Study 1 evaluated the immunogenicity of DTaP-IPV/Hib, PCV13, HepB, MMR and varicella vaccine when given concomitantly with MenQuadfi compared to Menveo [see Adverse Reactions (6.1)]. The immune responses to DTaP-IPV/Hib, PCV13, and HepB were evaluated 1 month following Dose 3 of each of these vaccines. The immune responses to PCV13 and DTaP-IPV/Hib were evaluated 1 month after Dose 4 of each of these vaccines. The immune responses to MMR and varicella vaccine were evaluated 1 month after vaccination. In addition, the immune response to DTaP-IPV/Hib when given concomitantly with MenQuadfi at 15 through 18 months of age was compared to DTaP-IPV/Hib given alone at 15 through 18 months.
No interference in immune responses to these vaccines was observed when they were given concomitantly with MenQuadfi.
Concomitant administration of MenQuadfi with Tdap and HPV in adolescents 10 through 17 years was evaluated in Study 5 (NCT02199691). In this randomized study, 503 participants received MenQuadfi alone, 392 received MenQuadfi coadministered with Tdap and HPV, 296 received Tdap and HPV alone. A fourth group received Menveo alone (N=501).
No evidence of interference in hSBA seroresponse rates was observed when MenQuadfi was coadministered with Tdap and HPV. Antibody responses to HPV, and to the tetanus and diphtheria antigens were similar when Tdap and HPV were administered with and without MenQuadfi. Anti-pertussis GMC responses were non-inferior for the pertussis toxoid antigen, but did not meet non-inferiority for the FHA, PRN, and FIM antigens. The clinical relevance of the diminished responses to the pertussis antigens is unknown.
16. How is MenQuadfi supplied
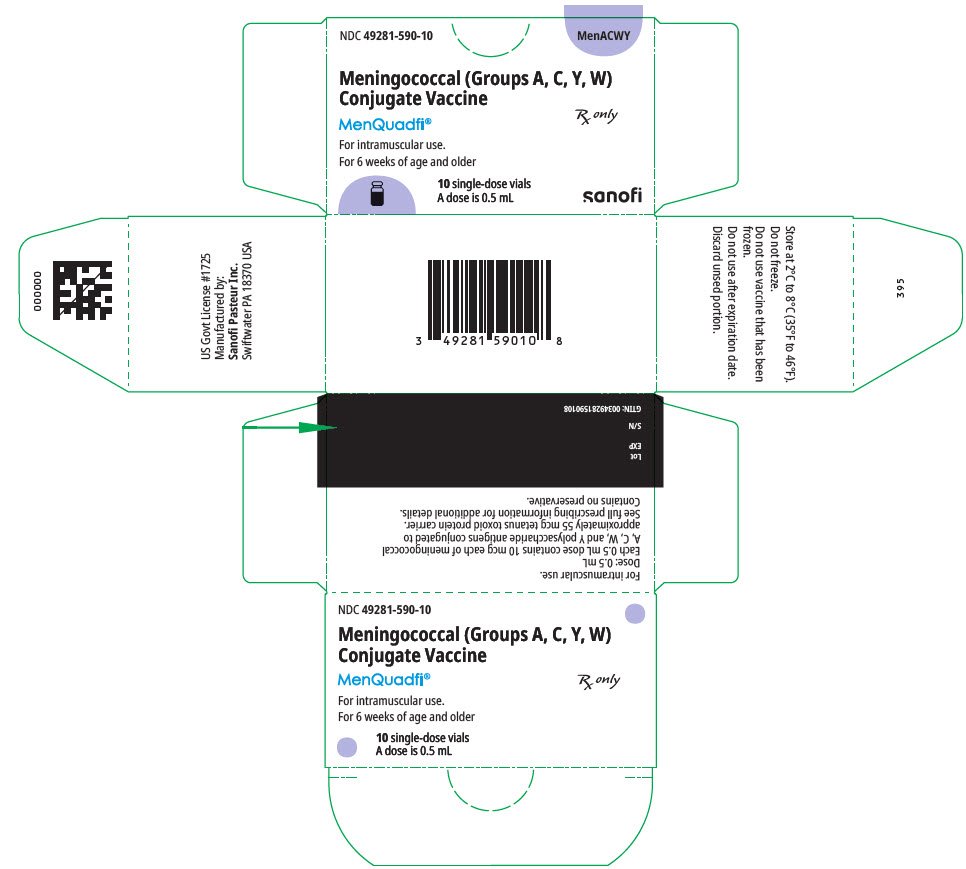
MenQuadfi is supplied in a single-dose vial (NDC 49281-590-58):
in packages of 1 vial (NDC 49281-590-01);
in packages of 5 vials (NDC 49281-590-05);
in packages of 10 vials (NDC 49281-590-10).
Not all pack sizes may be marketed.
The vial stopper is not made with natural rubber latex.
17. Patient Counseling Information
Vaccine Information Statements are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization to the patient, parent, or guardian. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines). Inform the patients, parents or guardians about:
- Potential benefits and risks of immunization with MenQuadfi.
- Potential for adverse reactions that have been temporally associated with administration of MenQuadfi or other vaccines containing similar components.
- Reporting any adverse reactions to their healthcare provider.
- The Sanofi Pasteur Inc. Pregnancy Registry, as appropriate [see Pregnancy (8.1)].
MenQuadfi is a registered trademark of Sanofi Pasteur Inc.
Menactra, Adacel and Menomune are registered trademarks of Sanofi, its affiliates and/or its subsidiaries.
The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to Sanofi, its affiliates and/or its subsidiaries.
Manufactured by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
© 2025, Sanofi Pasteur Inc. - All rights reserved
R10-0525
| MENQUADFI
neisseria meningitidis group a capsular polysaccharide tetanus toxoid conjugate antigen, neisseria meningitidis group c capsular polysaccharide tetanus toxoid conjugate antigen, neisseria meningitidis group y capsular polysaccharide tetanus toxoid conjugate antigen, and neisseria meningitidis group w-135 capsular polysaccharide tetanus toxoid conjugate antigen injection, solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Registrant - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE(49281-590) , PACK(49281-590) , LABEL(49281-590) | |
More about MenQuadfi (meningococcal conjugate vaccine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- En español
Patient resources
- MenQuadfi drug information
- MenQuadfi (Meningococcal vaccine, tetanus toxoid conjugate quadrivalent Intramuscular) (Advanced Reading)