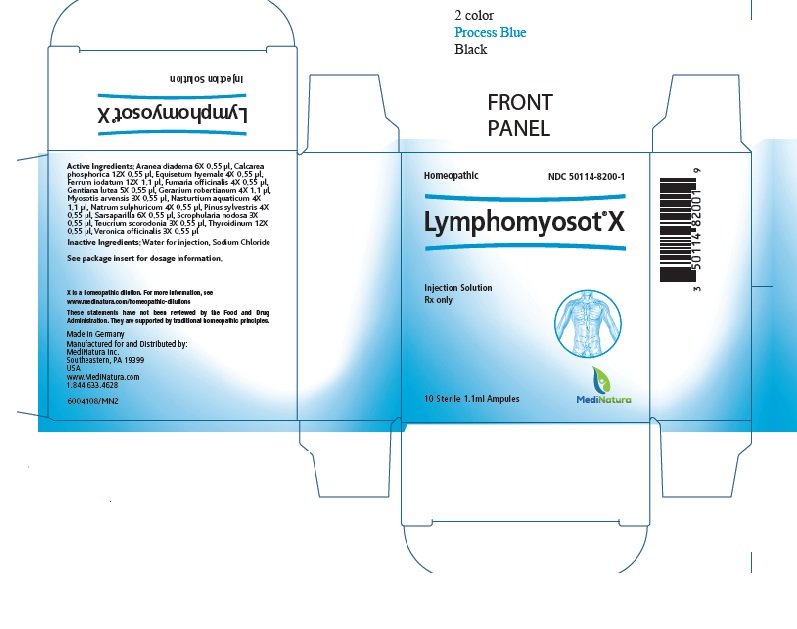
Lymphomyosot X: Package Insert / Prescribing Info
Package insert / product label
Dosage form: injection
On This Page
Lymphomyosot X Description
|
Ingredient name |
Potency |
Quantity |
Final dilution |
|
Aranea diadema |
6X |
0.55 μl |
9.30X |
|
Calcarea phosphorica |
12X |
0.55 μl |
15.30X |
|
Equisetum hyemale |
4X |
0.55 μl |
7.30X |
|
Ferrum iodatum |
12X |
1.1 μl |
15.00X |
|
Fumaria officinalis |
4X |
0.55 μl |
7.30X |
|
Gentiana lutea |
5X |
0.55 μl |
8.30X |
|
Geranium robertianum |
4X |
1.1 μl |
7.00X |
|
Myosotis arvensis |
3X |
0.55 μl |
6.30X |
|
Nasturtium aquaticum |
4X |
1.1 μl |
7.00X |
|
Natrum sulphuricum |
4X |
0.55 μl |
7.30X |
|
Pinus sylvestris |
4X |
0.55 μl |
7.30X |
|
Sarsaparilla |
6X |
0.55 μl |
9.30X |
|
Scrophularia nodosa |
3X |
0.55 μl |
6.30X |
|
Teucrium scorodonia |
3X |
0.55 μl |
6.30X |
|
Thyroidinum |
12X |
0.55 μl |
15.30X |
|
Veronica officinalis |
3X |
0.55 μl |
6.30X |
Indications and Usage for Lymphomyosot X
Lymphomyosot x® Injection Solution is a homeopathic drug product indicated for the improvement of lymphatic drainage, the non-specific immune defense, and conditions such as benign hypertrophy of lymphnodes, chronic tonsillitis, tonsillar hypertrophy and lymphatic edema.
Lymphomyosot X Dosage and Administration
General Consideration
• If co-administration with a local anesthetic is desired, Lymphomyosot® X Injection Solution may be mixed with lidocaine or similar agents at the discretion of the physician.
• The dosage schedules listed below can be used as a general guide for the administration of Lymphomyosot® X Injection Solution.
• Lymphomyosot® X Injection Solution may be administered s.c., i.d., i.m., or i.v.
• The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
• Parenteral drug products should be inspected visually for particulate matter and discoloration prior to
administration, whenever solution and container permit.
• Draw up required dose into syringe.
• Discard any unused ampule contents. Do not reuse ampule.
• Only licensed practitioners with sufficient expertise in injecting drugs, including the respective route of administration, should administer the product.
2.2 Standard Dosage:
Adults and children 12 years and older:
1 ml 1 to 3 times per 7 days.
Children 6 to 11 years:
0.7 ml 1 to 3 times per 7 days.
Children 2 to 5 years:
0.5 ml 1 to 3 times per 7 days.
2.3 Acute Dosage:
Adults and children 12 years and older:
1 ml daily, and then continue with standard dosage.
Children 6 to 11 years:
0.7 ml daily, and then continue with standard dosage.
Children 2 to 5 years:
0.5 ml daily, and then continue with standard dosage.
Contraindications
Lymphomyosot® X Injection Solution is contraindicationed in patients with known hypersensiticety to Lymphomyost® X or any of its ingredients.
Overdosage
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
Adverse Reactions/Side Effects
Post-marketing Experience
The following adverse events have been identified during post-marketing use of Lymphomyosot® X Injection Solution. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
• Allergic (hypersensitivity) skin reactions may occur in isolated cases.
To report SUSPECTED ADVERSE REACTIONS, contact MediNatura. at 1.844.633.4628 or info@medinatura.com or FDA at 1-800-FDA-1088
or www.fda.gov/medwatch.
Dosage Forms and Strengths
One ampule containing 1.1 ml solution for injection each containing the active ingredients in the strengths listed under Description.
| LYMPHOMYOSOT X
araneus diadematus, tribasic calcium phosphate, equisetum hyemale, ferrous iodide,fumaria officinalis flowering top, gentiana lutea root, geranium robertianum, myosotis arvensis, nasturtium officinale, sodium sulfate, pinus sylvestris leafy twig,smilax regelii root, scrophularia nodosa, teucrium scorodonia flowering top, thyroid, unspecified andveronica officinalis flowering top injection |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MediNatura (102783016) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hameln Pharma GmbH | 315869123 | manufacture(50114-8200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biologische Heilmittel Heel | 315635359 | manufacture(50114-8200) | |