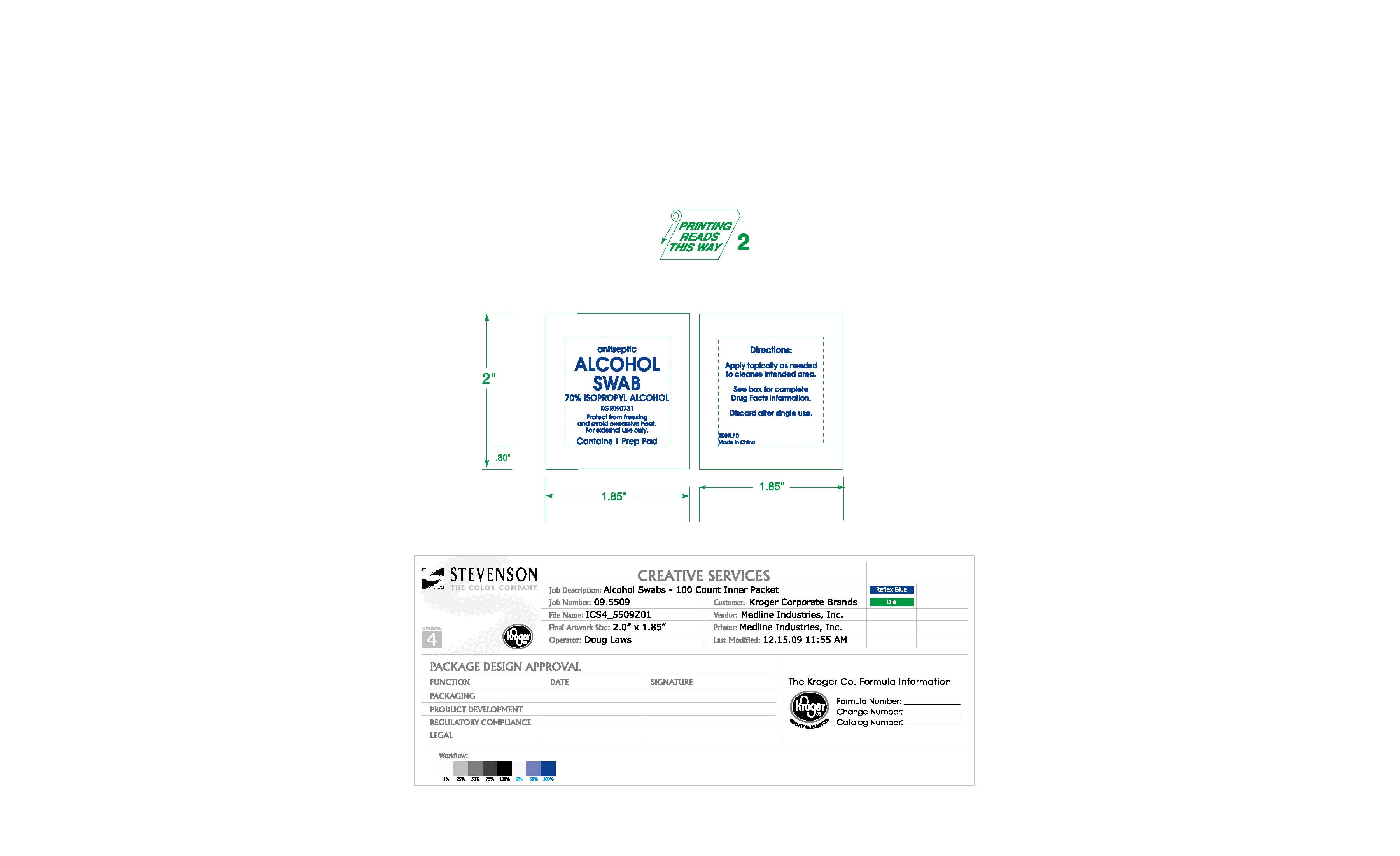
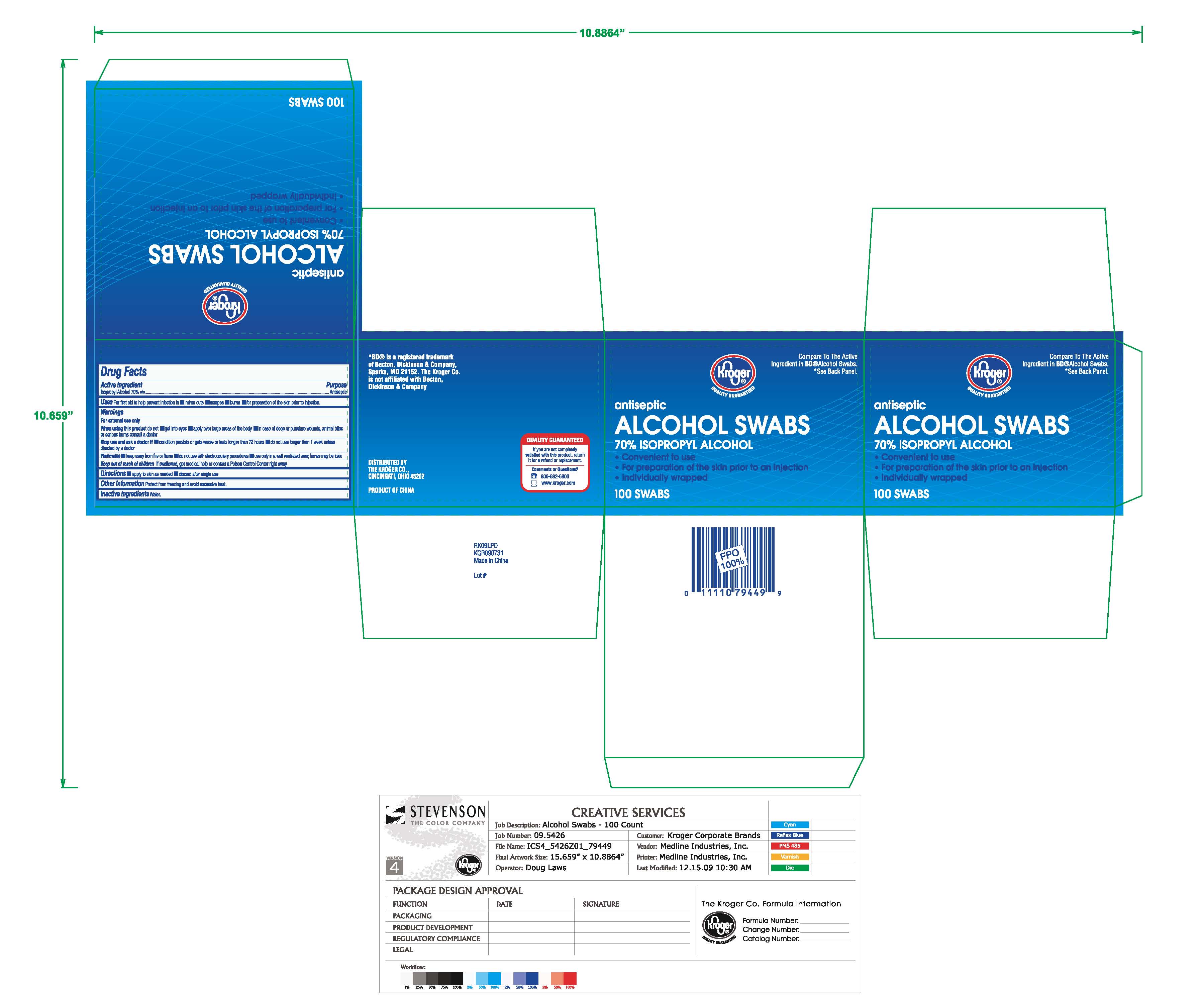
Isopropyl Alcohol Swabs: Package Insert / Prescribing Info
Package insert / product label
Dosage form: swab
Drug class: Antiseptic and germicides
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
Indications and Usage for Isopropyl Alcohol Swabs
For preparation of the skin prior to injection. For first aid to decrease germs in minor cuts, scrapes and burns.
Warnings
- For external use only.
- Flammable. Keep away from fire or flame.
- Use only in a well-ventilated area; fumes may be toxic.
When using this product do not
- get into eyes
- apply over large areas of the body
In case of deep or puncture wounds, animal bites or serious burns, consult a doctor.
Related/similar drugs
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control center right away.
| ISOPROPYL ALCOHOL
isopropyl alcohol swab |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - The Kroger Company (006999528) |
More about isopropyl alcohol topical
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Latest FDA alerts (3)
- Side effects
- Drug class: antiseptic and germicides
- En español