Inlexzo: Package Insert / Prescribing Info
Package insert / product label
Generic name: gemcitabine intravesical
Dosage form: intravesical system
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
INLEXZO™ (gemcitabine intravesical system)
Initial U.S. Approval: 2025
Indications and Usage for Inlexzo
INLEXZO is a nucleoside metabolic inhibitor-containing intravesical system, indicated for the treatment of adult patients with Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ(CIS) with or without papillary tumors. ( 1)
Inlexzo Dosage and Administration
For Intravesical Administration Only
- Insert INLEXZO (225 mg of gemcitabine) into the bladder once every 3 weeks up to 6 months (8 doses), followed by once every 12 weeks (6 doses). ( 2.2)
- Insert into the bladder using the co-packaged urinary catheter and stylet only. ( 2.1)
- Remove INLEXZO after each 3-week indwelling period. ( 2.2)
- See Full Prescribing Information and Instructions for Use for insertion and removal procedures. ( 2.3)
Dosage Forms and Strengths
- One single-dose 225 mg gemcitabine intravesical system ( 3)
Contraindications
Warnings and Precautions
- Risks in Patients with Perforated Bladder: Evaluate the bladder before the intravesical insertion of INLEXZO. Do not administer to patients with a perforated bladder or in whom the integrity of the bladder mucosa has been compromised. ( 4, 5.1)
- Risk of Metastatic Bladder Cancer with Delayed Cystectomy: Delaying cystectomy can lead to the development of metastatic bladder cancer, which can be lethal. ( 5.2)
- Magnetic Resonance Imaging (MRI) Safety: INLEXZO can only be safely scanned with MRI under certain conditions. ( 5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and to use effective contraception. ( 5.4, 8.1, 8.3)
Adverse Reactions/Side Effects
- The most common (>15%) adverse reactions, including laboratory abnormalities, are urinary frequency, urinary tract infection, dysuria, micturition urgency, decreased hemoglobin, increased lipase, urinary tract pain, decreased lymphocytes, hematuria, increased creatinine, increased potassium, increased AST, decreased sodium, bladder irritation, and increased ALT. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
Use In Specific Populations
- Lactation: Advise not to breastfeed. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2025
Full Prescribing Information
1. Indications and Usage for Inlexzo
INLEXZO is indicated for the treatment of adult patients with Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ(CIS), with or without papillary tumors.
2. Inlexzo Dosage and Administration
2.1 Important Administration Instructions
Administer INLEXZO intravesically only. Do NOT administer by any other route. INLEXZO is co-packaged with a urinary catheter and stylet used to insert INLEXZO through the urinary catheter into the bladder. Administer using the co-packaged urinary catheter and stylet only.
INLEXZO should be inserted and removed by a trained healthcare provider. Healthcare providers should become thoroughly familiar with the insertion and removal instructions before attempting insertion or removal of INLEXZO.
Prophylactic antibiotics may be used at the discretion of the treating healthcare provider with each INLEXZO insertion and removal.
2.2 Recommended Dosage
Insert INLEXZO (225 mg of gemcitabine) into the bladder once every 3 weeks for up to 6 months (8 doses), followed by once every 12 weeks for up to 18 months (6 doses), or until persistent or recurrent NMIBC, disease progression, or unacceptable toxicity.
Remove INLEXZO after each 3-week indwelling period.
Missed Dose
If a dose is missed, it should be administered as closely as possible to the original treatment schedule.
2.3 Preparation and Intravesical Administration
See the Instructions for Useenclosed in the carton for complete information on preparation, intravesical administration, and removal of INLEXZO.
INLEXZO is a hazardous drug. Follow applicable special handling and disposal procedures while handling INLEXZO and during the insertion and removal procedure. 1
Instruct patients to drink approximately 1500 mL of fluids per day during therapy with INLEXZO to ensure adequate urine production for drug release.
Instruct patients not to empty the bladder immediately prior to the insertion procedure. Presence of urine in the bladder can facilitate deployment of INLEXZO. Patients can resume micturition after the insertion procedure.
During indwelling period of approximately 3 weeks, advise patients to avoid urine contact with skin, to void urine sitting on a toilet, to wash hands with soap and water and to wash their genital area with water after each urination, and to flush the toilet after use.
3. Dosage Forms and Strengths
One single-dose 225 mg strength gemcitabine intravesical system consisting of a flexible bi-oval shaped tube containing an almost white to light pink-brown colored component at the center surrounded by off white to light blue components.
4. Contraindications
INLEXZO is contraindicated in patients with:
- Perforation of the bladder [see Warnings and Precautions (5.1)] .
- Prior hypersensitivity reactions to gemcitabine or any component of the product.
5. Warnings and Precautions
5.1 Risks in Patients with Perforated Bladder
INLEXZO may lead to systemic exposure to gemcitabine and to severe adverse reactions if administered to patients with a perforated bladder or to those in whom the integrity of the bladder mucosa has been compromised.
Evaluate the bladder before the intravesical administration of INLEXZO and do not administer to patients with a perforated bladder or mucosal compromise until bladder integrity has been restored [see Contraindications (4)].
5.2 Risk of Metastatic Bladder Cancer with Delayed Cystectomy
Delaying cystectomy in patients with BCG-unresponsive CIS could lead to development of muscle invasive or metastatic bladder cancer, which can be lethal. The risk of developing muscle invasive or metastatic bladder cancer increases the longer cystectomy is delayed in the presence of persisting CIS.
Of the 83 evaluable patients with BCG-unresponsive CIS treated with INLEXZO in Cohort 2 of SunRISe-1, seven patients (8%) progressed to muscle invasive (T2 or greater) bladder cancer. Three patients (3.5%) had progression determined at the time of cystectomy. The median time between determination of persistent or recurrent CIS or T1 and progression to muscle invasive disease was 94 days.
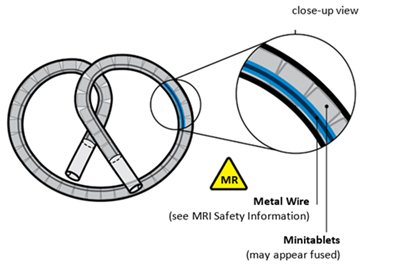
5.3 Magnetic Resonance Imaging (MRI) Safety
INLEXZO may be used with MRI only under the specific predefined conditions provided below to avoid potential safety hazards or severe adverse reactions.
Based on clinical experience in patients treated with INLEXZO indwelling in the bladder who underwent MRI scans and nonclinical testing, INLEXZO is MR Conditional. A patient with INLEXZO indwelling in the bladder can be scanned in an MR system under the following conditions:
- Static magnetic field of 1.5-Tesla and 3-Tesla, only.
- Maximum spatial gradient magnetic field of 5000 Gauss/cm or less.
- Maximum magnetic resonance system reported, whole body averaged specific absorption rate of 2-W/kg for 60 minutes of continuous scanning (i.e., per pulse sequence or back-to-back sequences without breaks) in the Normal Operating Mode of operation for the MR system.
Under the scan conditions defined, INLEXZO is expected to produce a maximum temperature rise of 2°C after 15 minutes of continuous scanning.
In nonclinical testing, the image artifact caused by INLEXZO extends approximately 2 mm from INLEXZO using a gradient echo pulse sequence and a 3-Tesla MR system.
5.4 Embryo-Fetal Toxicity
Based on animal data and its mechanism of action, INLEXZO can cause fetal harm when administered to a pregnant woman if systemic exposure occurs [see Clinical Pharmacology (12.1)] . In animal reproduction studies, systemic administration of gemcitabine was teratogenic, embryotoxic, and fetotoxic in mice and rabbits.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after final removal of INLEXZO. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after final removal of INLEXZO [see Use in Specific Populations (8.1, 8.3)] .
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of INLEXZO monotherapy was evaluated in Cohort 2 of SunRISe-1, a multi-center, open-label study in 85 adult patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors [see Clinical Studies (14.1)].
Patients received INLEXZO (225 mg of gemcitabine) inserted into the bladder every 3 weeks for 6 months, followed by once every 12 weeks for up to 18 months, or until unacceptable toxicity, disease persistence, recurrence, or progression [see Dosage and Administration (2.2)] .
The median number of doses of INLEXZO administered to patients was 9 (range: 1 to 14) doses. The median duration of exposure to INLEXZO was 41 weeks (range: 1 to 108 weeks).
Serious adverse reactions occurred in 24% of patients receiving INLEXZO. Serious adverse reactions that occurred in >2% of patients included urinary tract infection, hematuria, pneumonia, and urinary tract pain. Fatal adverse reactions occurred in 1.2% of patients who received INLEXZO, including cognitive disorder.
Permanent discontinuation of INLEXZO due to an adverse reaction occurred in 7% of patients. Adverse reactions which resulted in permanent discontinuation of INLEXZO in >1% of patients included bladder irritation, urinary frequency, cognitive disorder, hydronephrosis, and urinary tract disorder.
Dosage interruptions of INLEXZO due to an adverse reaction occurred in 41% of patients. Adverse reactions which required dosage interruption in >3% of patients included urinary tract infection, urinary tract pain, hematuria, urinary frequency, micturition urgency, dysuria, and genital pain.
The most common (>15%) adverse reactions, including laboratory abnormalities, were urinary frequency, urinary tract infection, dysuria, micturition urgency, decreased hemoglobin, increased lipase, urinary tract pain, decreased lymphocytes, hematuria, increased creatinine, increased potassium, increased AST, decreased sodium, bladder irritation, and increased ALT.
Table 1 summarizes the adverse reactions in SunRISe-1.
| Adverse Reaction | INLEXZO
N=85 |
|
|---|---|---|
| All Grades
% | Grade 3 or 4
% |
|
|
||
| Urinary frequency | 48 | 0 |
| Urinary tract infection * | 44 | 6 |
| Dysuria | 42 | 0 |
| Micturition urgency * | 34 | 0 |
| Urinary tract pain * | 26 | 7 |
| Hematuria * | 24 | 2.4 |
| Bladder irritation * | 16 | 0 |
Other clinically significant adverse reactions (<15%) included fatigue (14%), genital pain (12%), diarrhea (11%), urinary incontinence (9%), urinary retention (7%), and nocturia (4.7%).
| Laboratory Abnormality | INLEXZO * | |
|---|---|---|
| All Grades
(%) | Grade 3 or 4
(%) |
|
|
||
| Hematology | ||
| Decreased Hemoglobin | 31 | 1.2 |
| Decreased Lymphocytes | 24 | 4.8 |
| Chemistry | ||
| Increased Lipase | 28 | 12 |
| Increased Creatinine | 24 | 0 |
| Increased Potassium | 22 | 1.2 |
| Increased AST | 17 | 1.2 |
| Decreased Sodium | 16 | 4.8 |
| Increased ALT | 16 | 1.2 |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on animal data and its mechanism of action, INLEXZO can cause fetal harm when administered to a pregnant woman if systemic exposure occurs [see Clinical Pharmacology (12.1)] . There are no available data on the use of INLEXZO in pregnant women to inform a drug-associated risk. In animal reproduction studies, systemic administration of gemcitabine was teratogenic, embryotoxic, and fetotoxic in mice and rabbits (see Data) . Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
Gemcitabine is embryotoxic in mice. Daily systemic dosing of gemcitabine to pregnant mice increased the incidence of fetal malformation (cleft palate, incomplete ossification) at doses of 1.5 mg/kg/day. Gemcitabine was embryotoxic and fetotoxic in rabbits. Daily systemic dosing of gemcitabine to pregnant rabbits resulted in fetotoxicity (decreased fetal viability, reduced litter sizes, and developmental delays) and increased the incidence of fetal malformations (fused pulmonary artery, absence of gall bladder) at doses of 0.1 mg/kg/day.
8.2 Lactation
Risk Summary
There is no information regarding the presence of gemcitabine or its metabolites in human milk, or their effects on the breastfed infant or milk production following INLEXZO administration. Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment and for 1 week after final removal of INLEXZO.
8.3 Females and Males of Reproductive Potential
INLEXZO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating INLEXZO.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment and for 6 months after final removal of INLEXZO.
Males
Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 months after final removal of INLEXZO [see Nonclinical Toxicology (13.1)] .
Infertility
Males
Based on animal studies, INLEXZO may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)] . It is not known whether these effects on fertility are reversible.
8.4 Pediatric Use
Safety and effectiveness of INLEXZO in pediatric patients have not been established.
8.5 Geriatric Use
Of the patients given INLEXZO monotherapy in Cohort 2 of SunRISe-1, 72% were 65 years of age or older and 34% were 75 years or older. There were insufficient numbers of patients <65 years of age to determine if these patients respond differently to patients 65 years of age and older.
11. Inlexzo Description
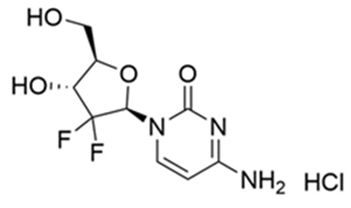
INLEXZO contains gemcitabine hydrochloride, a nucleoside metabolic inhibitor. Gemcitabine hydrochloride is 2'-deoxy-2',2'- difluorocytidine monohydrochloride (β-isomer) with a molecular formula of C9H11F2N3O4 ∙ HCl, and a molecular weight of 299.66. The structural formula is:
INLEXZO is a sterile, non-resorbable intravesical system containing the equivalent of 225 mg gemcitabine (present as 256.3 mg of gemcitabine hydrochloride).
Gemcitabine Intravesical System
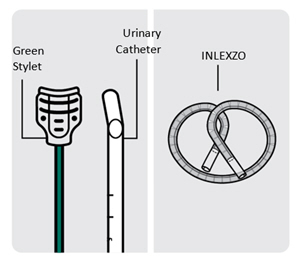
INLEXZO is a bi-oval-shaped tube containing an almost white to light pink-brown colored gemcitabine component at the center surrounded on each side by off white to light blue-colored osmotic components.
- The gemcitabine component contains 225 mg of gemcitabine and the following inactive ingredients: polyethylene glycol 8000 (8.0 mg), povidone K30 (13.4 mg), and urea (42.6 mg).
- The osmotic components contain the following inactive ingredients: FD&C Blue No.1 (0.0042 mg), polyethylene oxide 600,000 (72.0 mg), and urea (648.0 mg).
The silicone tube contains two lumens, the larger one containing the drug components and silicone spacers, and the smaller one containing a superelastic nitinol wire in a predefined shape (wireform). Both lumens are capped with a silicone adhesive. The lumen containing the gemcitabine and osmotic components has a single delivery orifice. INLEXZO's coiled dimensions are approximately 5.5 cm wide × 4.5 cm high.
Urinary Catheter and Stylet
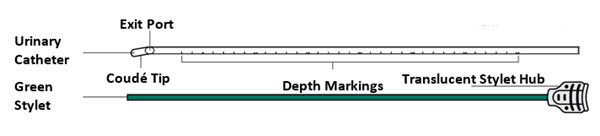
INLEXZO is co-packaged with a sterile urinary catheter and a sterile stylet, required for transurethral insertion into the bladder. The urinary catheter and stylet are made of thermoplastic elastomer and polyethene, and consist of the following components:
- A semi-transparent urinary catheter, with a rounded blunt distal tip that includes a coudé tip, a product exit port near the distal tip, and a lumen which extends from the exit port to an open proximal end. The outer diameter of the urinary catheter is 5.82 mm (17.5 Fr). Printed depth markings are placed on the urinary catheter to indicate insertion depth and orientation of the coudé tip to assist the user during insertion.
- A green stylet with a hub at the proximal end is used to advance INLEXZO through the urinary catheter lumen and into the bladder. The stylet length and proximal hub prevent the stylet's distal end from advancement beyond the exit port.
12. Inlexzo - Clinical Pharmacology
12.1 Mechanism of Action
Gemcitabine kills cells undergoing DNA synthesis and blocks the progression of cells through the G1/S-phase boundary. Gemcitabine is metabolized by nucleoside kinases to diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides. Gemcitabine diphosphate inhibits ribonucleotide reductase, an enzyme responsible for catalyzing the reactions that generate deoxynucleoside triphosphates for DNA synthesis, resulting in reductions in deoxynucleotide concentrations, including dCTP. Gemcitabine triphosphate competes with dCTP for incorporation into DNA. The reduction in the intracellular concentration of dCTP by the action of the diphosphate enhances the incorporation of gemcitabine triphosphate into DNA (self-potentiation). After the gemcitabine nucleotide is incorporated into DNA, only one additional nucleotide is added to the growing DNA strands, which eventually results in the initiation of apoptotic cell death.
12.2 Pharmacodynamics
The exposure-response relationship and time-course of pharmacodynamic response for the safety and effectiveness of INLEXZO have not been fully characterized.
12.3 Pharmacokinetics
The systemic exposure of gemcitabine and the inactive uracil metabolite (2´-deoxy-2´,2´-difluorouridine [dFdU]), were evaluated between Days 2 and 7 during the indwelling period. The plasma gemcitabine concentrations were below the lower limit of quantification (0.1 µg/mL) in all patients at all time points. Eighteen patients (17%) had at least one quantifiable plasma dFdU concentration above the lower limit of quantification (0.1 µg/mL) and the maximum observed plasma dFdU concentration was 0.4 µg/mL. Plasma concentrations of both gemcitabine and dFdU are estimated to be less than 1% of the expected C maxafter intravenous administration of gemcitabine.
Excretion
Gemcitabine and dFdU are excreted in urine throughout the indwelling period for INLEXZO. Of the total gemcitabine dose, 77% was excreted by Day 7 and 99% was excreted by Day 21 in urine as gemcitabine and dFdU.
Mean urinary excretion on Days 2 through 5 ranged from 21 to 32 mg per day excreted in the urine as gemcitabine and dFdU.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of gemcitabine have not been conducted. Gemcitabine was mutagenic in an in vitromouse lymphoma (L5178Y) assay and was clastogenic in an in vivomouse micronucleus assay.
Dedicated animal fertility studies have not been conducted with gemcitabine intravesical system. Gemcitabine intraperitoneal doses of 0.5 mg/kg/day in male mice resulted in moderate to severe hypospermatogenesis, decreased fertility, and decreased implantations. In female mice, fertility was not affected but maternal toxicities were observed at 1.5 mg/kg/day administered intravenously and fetotoxicity or embryo lethality were observed at 0.25 mg/kg/day administered intravenously.
14. Clinical Studies
14.1 BCG-unresponsive NMIBC
The efficacy of INLEXZO was evaluated in Cohort 2 of SunRISe-1 (NCT04640623), a single-arm, multi-center trial in 83 adults with BCG-unresponsive, NMIBC with CIS, with or without papillary tumors (T1, or high-grade Ta) following transurethral resection.
BCG-unresponsive NMIBC CIS was defined as persistent or recurrent CIS alone or with Ta/T1 disease within 12 months of adequate BCG therapy. Adequate BCG therapy was defined as a minimum administration of at least five of six doses of an initial induction course plus either of: at least two of three doses of maintenance therapy or at least two of six doses of a second induction course. Prior to treatment, all patients had undergone transurethral resection of bladder tumor (TURBT) to remove all resectable disease (Ta and T1 components). Residual CIS (Tis components) not amenable to complete resection was permitted. The trial included patients who were ineligible for or who had elected not to undergo radical cystectomy and excluded patients with extra-vesical (i.e., urethra, ureter, or renal pelvis), muscle invasive (T2-T4), locally advanced, or metastatic urothelial carcinoma.
Patients received INLEXZO (225 mg of gemcitabine) into the bladder every 3 weeks for 6 months, followed by once every 12 weeks for up to 18 months, or until unacceptable toxicity, persistence or recurrence of CIS and/or high-grade papillary disease, or progression [see Dosage and Administration (2.2)]. Tumor status was assessed every 12 weeks during the initial two years of treatment, after which cystoscopy was performed at least every 24 weeks. Mandatory biopsies were performed 24 and 48 weeks after treatment initiation.
The major efficacy outcome measures were complete response rate at any time (defined as negative results for cystoscopy [with TURBT and centrally-reviewed biopsies as applicable] and centrally-reviewed urine cytology) and duration of response.
The median age of patients was 71 years (range: 40 to 88); 80% male; 87% White, 10% Asian, 2.4% Black or African American and 1.2% race not reported; 10% were of Hispanic or Latino ethnicity, 89% identified as not Hispanic or Latino, and 1.2% were ethnicity unknown or not reported. Baseline ECOG performance status was 0 (92%) or 1 (8%). Tumor pattern at study entry was CIS only (67%), CIS with high-grade Ta only (22%), and CIS with T1 (11%). All patients had predominant urothelial carcinoma including 1 patient (1.2%) with squamous differentiation. The median number of prior instillations of BCG was 12 (range: 7 to 42).
Efficacy results are summarized in Table 3.
| Endpoint | INLEXZO
N=83 |
|---|---|
| CI= confidence interval.
+ Denotes ongoing response |
|
|
|
| Complete Response Rate (95% CI) | 82% (72, 90) |
| Duration of Response * | |
| Range in months | 0+, 44+ |
| % (n) with duration ≥12 months | 51% (35) |
15. References
- OSHA Hazardous Drugs. Occupational Safety and Health Administration. Available at: https://www.osha.gov/hazardous-drugs (Accessed: 06 September 2024).
16. How is Inlexzo supplied
How Supplied
INLEXZO (gemcitabine intravesical system) contains 225 mg gemcitabine.
INLEXZO (NDC# 57894-225-01) is available in a carton containing:
- One sterile single-dose of INLEXZO in two clear laminate sleeves and packaged in an inner pouch. The inner pouch and a desiccant are packaged in an outer foil pouch. The outside surfaces of the inner and outer pouches are not sterile.
- One sterile urinary catheter and one sterile stylet packaged together in a pouch.
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Patient Information).
Risk of Metastatic Bladder Cancer with Delayed Cystectomy
- Inform patients that delaying cystectomy could lead to development of metastatic bladder cancer. Discuss the risk of metastatic bladder cancer and that the risk increases the longer cystectomy is delayed in the presence of persistent CIS [see Warnings and Precautions (5.2)] .
Magnetic Resonance Imaging (MRI) Safety
- Inform patients that INLEXZO can only be safely scanned with MRI under specific conditions [see Warnings and Precautions (5.3)] . Instruct patients who will have an MRI to tell their healthcare provider that they have INLEXZO.
- This information is included in the MRI Safety Information Card. Complete the MRI Safety Information Card and give it to the patient.
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4)and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment and for 6 months after final removal of INLEXZO [see Use in Specific Populations (8.3)] .
- Advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 months after final removal of INLEXZO [see Use in Specific Populations (8.3)].
Lactation
- Advise women not to breastfeed during treatment and for 1 week after final removal of INLEXZO [see Use in Specific Populations (8.2)].
Infertility
- Advise males of reproductive potential that INLEXZO may impair fertility [see Use in Specific Populations (8.3)] .
Important Post-Treatment Instructions
- Instruct patients not to empty the bladder immediately prior to the insertion procedure.
- Advise patients to avoid contact with urine while INLEXZO is indwelling in the bladder and for at least 24 hours post-removal [see Clinical Pharmacology (12.3)] .
- Advise patients to avoid urine contact with skin by voiding sitting on a toilet, flushing the toilet after use, and to wash hands with soap and water and to wash their genital area with water after each urination.
- Advise patients to wash clothing soiled with urine promptly and separately from other clothing [see Dosage and Administration (2.3)].
Manufactured for:
Janssen Biotech, Inc.
Horsham, PA 19044, USA
U.S. License Number 1864
For patent information: www.janssenpatents.com
© Johnson & Johnson and its affiliates 2025
| PATIENT INFORMATION
INLEXZO™ (inn-lex-zo) (gemcitabine intravesical system) |
||
|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issued: 09/2025 | |
What is INLEXZO?
|
||
Do not receive INLEXZO if you:
|
||
Before receiving INLEXZO, tell your healthcare provider about all of your medical conditions, including if you:
|
||
How will I receive INLEXZO?
|
||
| What should I avoid after INLEXZO is inserted?
Tell your healthcare provider that you have INLEXZO before having a type of scan called Magnetic Resonance Imaging (MRI). |
||
| INLEXZO may be used under specific conditions.Your healthcare provider will give you an MRI Safety Information Card. Keep the card in a safe place and show it to all of your healthcare providers. The card contains important information in case you need to have an MRI. Your healthcare provider will review the information on the MRI Safety Information Card and determine the conditions they can safely do an MRI scan while INLEXZO is in your bladder. | ||
| What are the possible side effects of INLEXZO?
The most common side effects of INLEXZO include: |
||
|
|
|
| INLEXZO may cause fertility problems in males, which may affect your ability to have children. It is unknown if these side effects of fertility are reversible. Talk to your healthcare provider if this is a problem for you.
Tell your healthcare provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of INLEXZO. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
| General information about the safe and effective use of INLEXZO.
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about INLEXZO that is written for healthcare professionals. |
||
| What are the ingredients in INLEXZO?
Active ingredient:gemcitabine hydrochloride Inactive ingredients for gemcitabine component:polyethylene glycol 8000, povidone K30, and urea. Inactive ingredients for osmotic components:FD&C Blue No.1, polyethylene oxide 600,000, and urea. Manufactured for: Janssen Biotech, Inc. Horsham, PA 19044, USA U.S. License Number 1864 For patent information: www.janssenpatents.com © Johnson & Johnson and its affiliates 2025 For more information, call 1-800-526-7736 or go to www.INLEXZO.com |
||
INSTRUCTIONS FOR USE
INLEXZO™
[inn-lex-zo]
(gemcitabine intravesical system)
For Intravesical Administration Only

Intended Use
INLEXZO releases gemcitabine into bladder urine during the indwelling period. See INLEXZO Prescribing Information (PI) for additional details.
The contents of the Instructions for Use provide instructions for both the insertion and removal of INLEXZO. Ensure that the Instructions for Use are available and reviewed prior to insertion and removal of INLEXZO.
User
INLEXZO should be inserted and removed by a trained healthcare provider.
 | MRI Safety Information
INLEXZOis MR Conditional. A patient with INLEXZOmay be safely scanned under the following conditions. Failure to follow these conditions may result in injury to the patient. |
|
| Parameter | Condition of Use/Information | |
| Nominal Values of Static Magnetic Field (T) | 1.5-Tesla and 3.0-Tesla | |
| Maximum Spatial Field Gradient (T/m and gauss/cm) | 50-T/m (5,000-gauss/cm) | |
| Type of RF Excitation | Circularly Polarized (CP) (i.e., Quadrature Transmission) | |
| Transmit RF Coil | Any transmit RF coil may be used. | |
| Receive RF Coil | Any receive-only RF coil may be used | |
| Maximum Whole Body Averaged SAR (W/kg) | 2-W/kg (Normal Operating Mode) | |
| Limits on Scan Duration | Whole body averaged SAR of 2-W/kg for 60 minutes of continuous RF exposure (i.e., per pulse sequence or back-to-back sequences/series without breaks) | |
| MR Image Artifact | The presence of INLEXZO produces an imaging artifact. Therefore, carefully select pulse sequence parameters if INLEXZO is located in the area of interest. | |
 | Precautions |
INLEXZO is a hazardous drug. Follow applicable special handling and disposal procedures while handling INLEXZO and during the insertion and removal procedure. Dispose of the used urinary catheter and stylet, INLEXZO, and its packaging per facility procedures and per applicable federal, state, and local regulations.
Wear gloves, and take appropriate precautions, per local guidelines for handling hazardous drugs,to prevent skin or mucus membrane exposure while handling INLEXZO and during the insertion and removal procedure.
If contact with INLEXZO is suspected, immediately wash the skin thoroughly or rinse the mucosa with copious amounts of water.
Advise patients and caregivers to exercise caution when handling urine during indwelling period of approximately 3 weeks. See INLEXZO PI for details.
INLEXZO contains one single-dose 225 mg strength gemcitabine intravesical system consisting of a flexible bi-oval shaped tube containing an almost white to light pink-brown colored component at the center surrounded by off white to light blue osmotic components.
Important Information
Use aseptic technique during insertion and removal of INLEXZO.
Follow these instructions carefully, to avoid patient injury and ensure proper functioning.
Insertion
To ensure proper insertion of INLEXZO and to avoid damage to INLEXZO, use only the following:
- Water-based lubricant
- Urinary catheter and stylet (supplied)
Do notuse the urinary catheter and stylet for any other purpose. Do not re-sterilize/re-use the urinary catheter or stylet. Re-use of the urinary catheter and stylet can lead to its degradation, failure, and contamination, which can increase the risk of infection or transmission of blood borne pathogens to patients and users.
Do notuse any components that are damaged or have damaged packaging.
Check the expiration ('EXP') date before use.
Do notuse INLEXZO if expiration date has passed.
Removal
To ensure proper INLEXZO removal and to avoid damage to INLEXZO and/or cystoscope, use only the following:
- Non-cutting, grasping forceps
- Flexible or rigid cystoscope
Storage
Store in the original package at 20°C to 25°C(68°F to 77°F); with excursions permitted between 15°C to 30°C(59°F to 86°F) [see USP Controlled Room Temperature].
INLEXZO™
Intravesical System
Urinary Catheter and Stylet
| Prepare components for INLEXZO insertion | ||
|---|---|---|
| 1. Gather supplies | ||
Included in the product carton:
| ||
| 2. Put on gloves | ||
 | 3. Prepare two syringes with sterile water-based lubricant
|
|
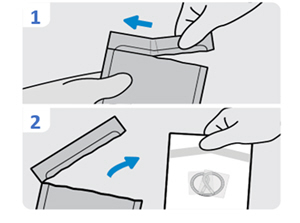 | 4. Open the INLEXZO foil pouch
|
|
 | 5. Examine the white component pouches for damage
Check the white INLEXZO pouch and white pouch containing the urinary catheter and stylet for damage (e.g., cuts, tears, punctures) that could compromise sterility of the components before opening. |
|
 | Do not use if packaging is damaged. | |
 | Surfaces of the white INLEXZO pouch and the white component pouches are not sterile. | |
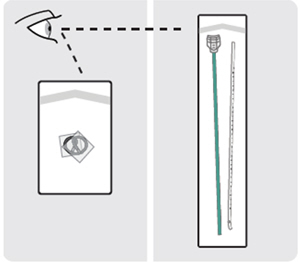
 | 6. Open the white component pouches and transfer the contents onto a sterile work surface
|
|
 | 7. Put on sterile gloves
|
|
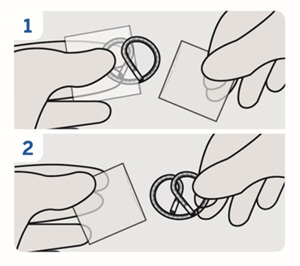 | 8. Remove plastic sleeves
Remove the plastic sleeves from INLEXZO. |
|
 | 9. Inspect components
Inspect INLEXZO, the urinary catheter, and green stylet for damage. Do notuse if the urinary catheter or green stylet are damaged or if the outer surface of INLEXZO is damaged. |
|
| Insert INLEXZO | ||
|---|---|---|
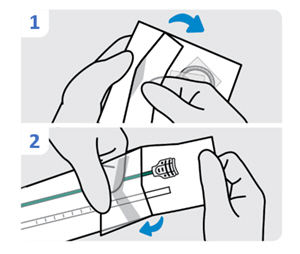
 | 1. Lubricate the urinary catheter
Lubricate tip of the urinary catheter. The urinary catheter should be free of INLEXZO and the stylet. |
|
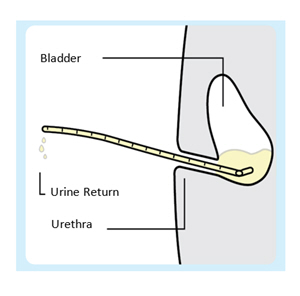
| 2. Insert the urinary catheter (without stylet)
Introduce the urinary catheter into the urethra by hand and advance until urine return. Do not empty the bladder. Use depth markings to maintain coudé tip orientation and insertion depth position throughout the procedure. |
||
 | Do not excessively force the urinary catheter into the bladder. | |
 | In case of resistance, careful assessment and standard procedural techniques can be performed, as appropriate, in order to safely advance the urinary catheter into the bladder. | |
 | If advancement is obstructed or cause cannot be determined or resolved, withdraw the urinary catheter to avoid patient injury or urinary catheter damage. | |
 | Do not re-use urinary catheter. | |
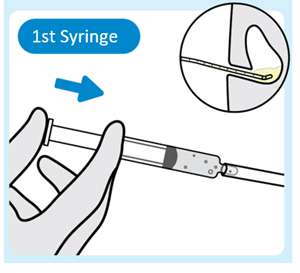 | 3. Inject lubricant from the first syringe into the urinary catheter
Inject 2 mL to 3 mL of lubricant from the first syringe into the end of the urinary catheter with the urinary catheter placed in the bladder. |
|
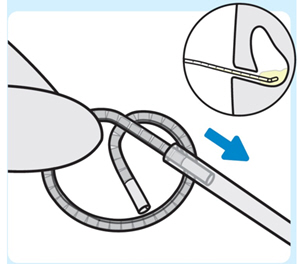 | 4. Insert INLEXZO into the urinary catheter
Insert either end of INLEXZO into the urinary catheter and advance until fully inserted. |
|
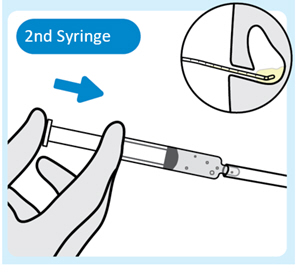 | 5. Inject lubricant from the second syringe into the urinary catheter
Inject 2 mL to 3 mL of lubricant from the second syringe into the urinary catheter to help advance INLEXZO further, with the urinary catheter placed in the bladder. |
|
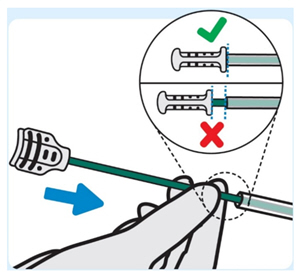 | 6. Insert stylet into the urinary catheter
Slowly insert the stylet into the urinary catheter until the stylet hub is flush with end of the urinary catheter. This ensures INLEXZO exits the urinary catheter and enters the bladder. |
|
 | If INLEXZO cannot be advanced, remove the urinary catheter and stylet together as a single unit. | |
| Ensure INLEXZO is also removed. | ||
| Do not attempt to re-use the removed INLEXZO. | ||
| Begin again by obtaining a new carton of INLEXZO including a new urinary catheter and stylet. | ||
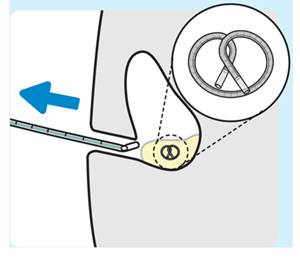 | 7. Remove the urinary catheter and stylet together as a single unit
Do notremove the urinary catheter and stylet individually. INLEXZO should remain inside the bladder. Retain Instructions for Use for the removal procedure. |
|
| Post-Insertion Instructions | |
|---|---|
 | 1. Provide the completed MRI Safety Information Card to the patient
Remove the MRI Safety Information Card from the carton. Complete the details and give it to the patient. Advise the patient to carry the card and to show their current and future healthcare providers in case of need for MRI scans.
|
| 2. Inform the patient and caregivers about the indwelling period | |
| The indwelling period is approximately 3 weeks. See INLEXZO PI for additional information. | |
| Inform the patient and caregivers that INLEXZO will remain in the bladder for the indwelling dosing period. | |
| INLEXZO contains a hazardous drug. The patient and caregivers should be made aware of the need to exercise caution when handling urine during the indwelling period. See INLEXZO PI for additional information. | |
| Removal of INLEXZO after the indwelling period | ||
|---|---|---|
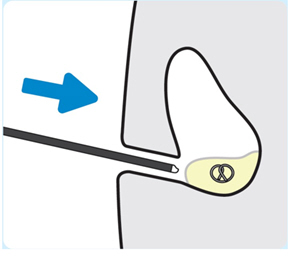 | 1. Lubricate the cystoscope
Use a water-based lubricant to lubricate the cystoscope. 2. Insert the cystoscope Insert the cystoscope into the bladder to locate INLEXZO. |
|
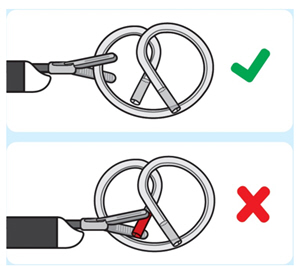 | 3. Grasp INLEXZO
Introduce non-cuttinggrasping forceps through the working channel of the cystoscope. |
|
 | Do not use cutting forceps. | |
| Grasp INLEXZO over tubing and metal wire. | ||
 | Do not grasp on or near the ends of INLEXZO. | |
| Grasping near the ends of INLEXZO could result in exposing the metal wire, potentially causing damage to surrounding tissue. | ||
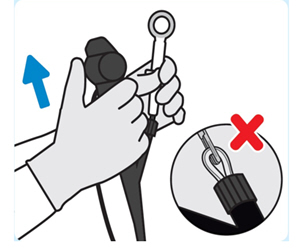 | 4. Remove INLEXZO
Remove the cystoscope and forceps out of the urethra togetherto remove INLEXZO under direct vision. Do notremove INLEXZO through working channel of the cystoscope. Doing so may damage INLEXZO and/or the cystoscope. |
|
| 5. Inspect INLEXZO | ||
 | After removal, inspect INLEXZO to confirm it is intact and unbroken. | |
Manufactured for:
Janssen Biotech, Inc.
Horsham, PA 19044, USA.
U.S. License Number 1864
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 09/2025
For patent information:
www.janssenpatents.com
© Johnson & Johnson and its affiliates 2025
PRINCIPAL DISPLAY PANEL - 225 mg Intravesical System Box
NDC 57894-225-01
inlexzo™
(gemcitabine intravesical system)
225 mg
For lntravesical Use Only
Contents:
- One sterile single-dose gemcitabine intravesical system
- One sterile urinary catheter (17.5 Fr) and one sterile green stylet
- One MRI Safety Information Card
- One Instructions for Use
- Prescribing Information
WARNING: Hazardous Drug
MR Conditional
Rx only
Johnson
&Johnson
Each intravesical system contains 256.3 mg gemcitabine
hydrochloride (equivalent to 225 mg gemcitabine free base).
Gemcitabine hydrochloride mini-tablets inactive ingredients:
polyethylene glycol 8000 (8.0 mg), povidone K30 (13.4 mg), and urea (42.6 mg).
Osmotic mini-tablets inactive ingredients: FD&C Blue No. 1 (0.0042 mg),
polyethylene oxide 600,000 (72.0 mg), and urea (648.0 mg).
Not made with natural rubber latex.
Recommended Dosage: See Prescribing Information.
Store in the original carton at 20 °C to 25 °C (68 °F to 77 °F); with excursions permitted
between 15 °C to 30 °C (59 °F to 86 °F) [see USP Controlled Room Temperature].
For additional assistance or to share your feedback on lnlexzo™,
call 1-800-526-7736.
Active Ingredient Made in India.
Manufactured for:
Janssen Biotech, Inc.
Horsham, PA 19044, USA
Pat. www.janssenpatents.com
Scan here for additional
product information.
Standard Data Fees Apply.
OPEN HERE

| INLEXZO
gemcitabine intravesical system |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Janssen Biotech, Inc (099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shilpa Pharma Lifesciences Limited | 650439131 | api manufacture(57894-225) , analysis(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shilpa Pharma Lifesciences Limited | 915076164 | analysis(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterigenics U.S., LLC | 883864076 | sterilize(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterigenics U.S., LLC | 030713940 | sterilize(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biolab Srl | 429117112 | analysis(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 370005019 | analysis(57894-225) , pack(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Johnson & Johnson Private Limited | 677603030 | analysis(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcami Carolinas Corporation | 117877975 | analysis(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 098908572 | pack(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BSP Pharmaceuticals SpA | 857007830 | analysis(57894-225) , manufacture(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Corden Pharma GmbH | 341627897 | analysis(57894-225) , manufacture(57894-225) , pack(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent CTS, LLC | 962674474 | analysis(57894-225) , manufacture(57894-225) , pack(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Labor LS SE & Co. KG | 314929072 | analysis(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Isomedix Operations, Inc. A STERIS Company | 080419356 | sterilize(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| STERIS Laboratories | 613214824 | sterilize(57894-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NACS, Inc. | 807781125 | manufacture(57894-225) | |
