Influenzinum: Package Insert / Prescribing Info
Package insert / product label
Generic name: influenza a virus a/michigan/45/2015 x-275 (h1n1) hemagglutinin antigen (formaldehyde inactivated), influenza a virus a/hong kong/4801/2014 x-263b (h3n2) hemagglutinin antigen (formaldehyde inactivated), influenza b virus b/brisbane/60/2008 hemagglutinin antigen (formaldehyde inactivated)
Dosage form: pellet
Active Ingredients
Influenzinum HPUS
8X, 30X, 6C, 9C, 12C, 15C, 30C, 200CK
Indications and Usage for Influenzinum
After-effects of flu or flu-like symptoms*
Warnings
Stop use and ask a doctor if
symptoms persist for more than 3 days or worsen.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Influenzinum Dosage and Administration
5 pellets 3 times a day until symptoms are relieved.
Other information
Do not use if pellet dispenser seal is broken.
contains approx. 80 pellets
Inactive ingredients
lactose, sucrose
Questions, Comments?
1-800-BOIRON-1
Newtown Square, PA 19073-3267
info@boironusa.com
*
These "Uses" have not been evaluated by the FDA.
Principle Display Panel - Influenzinum HPUS 8X
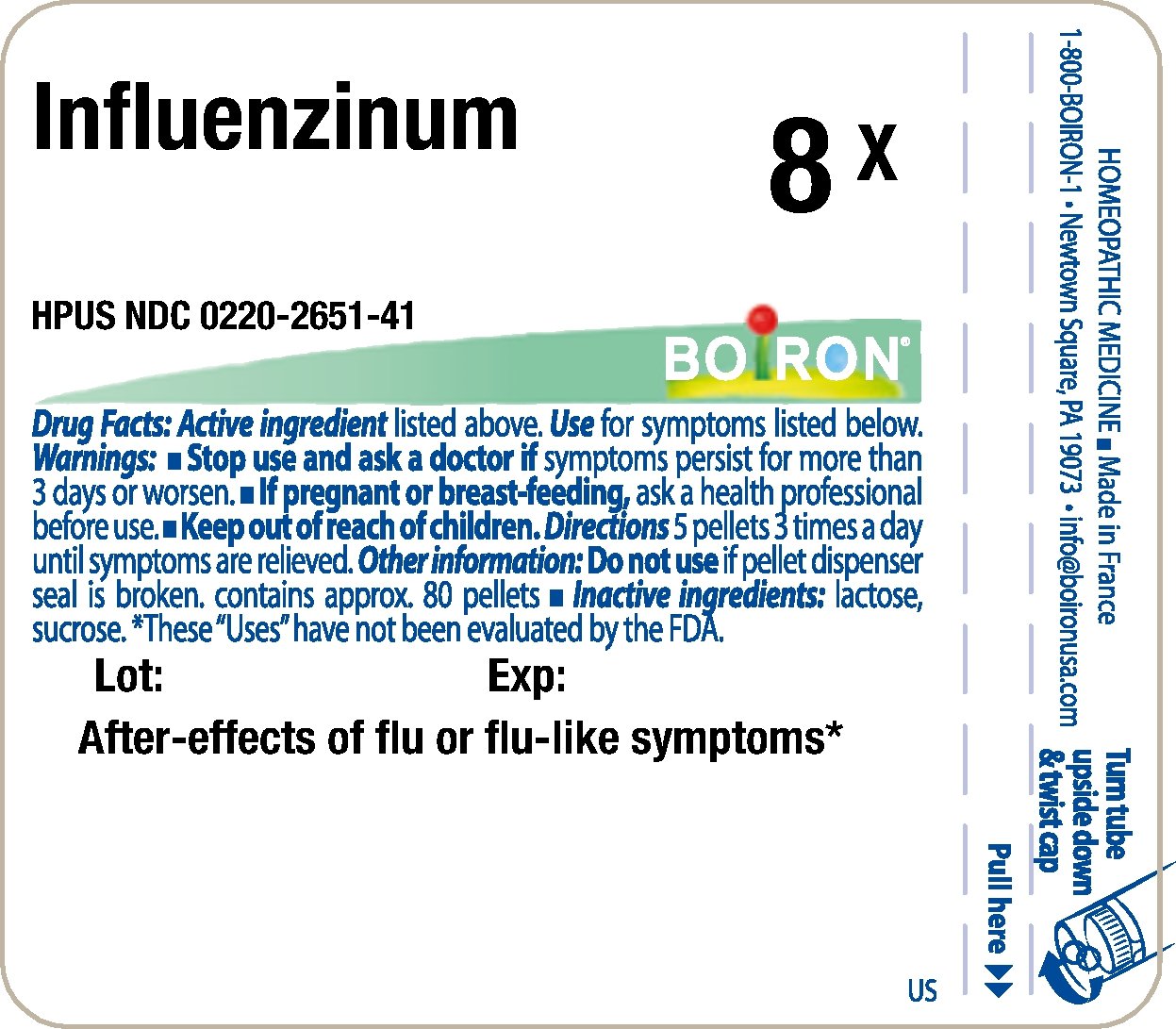
Principle Display Panel - Influenzinum HPUS 30X
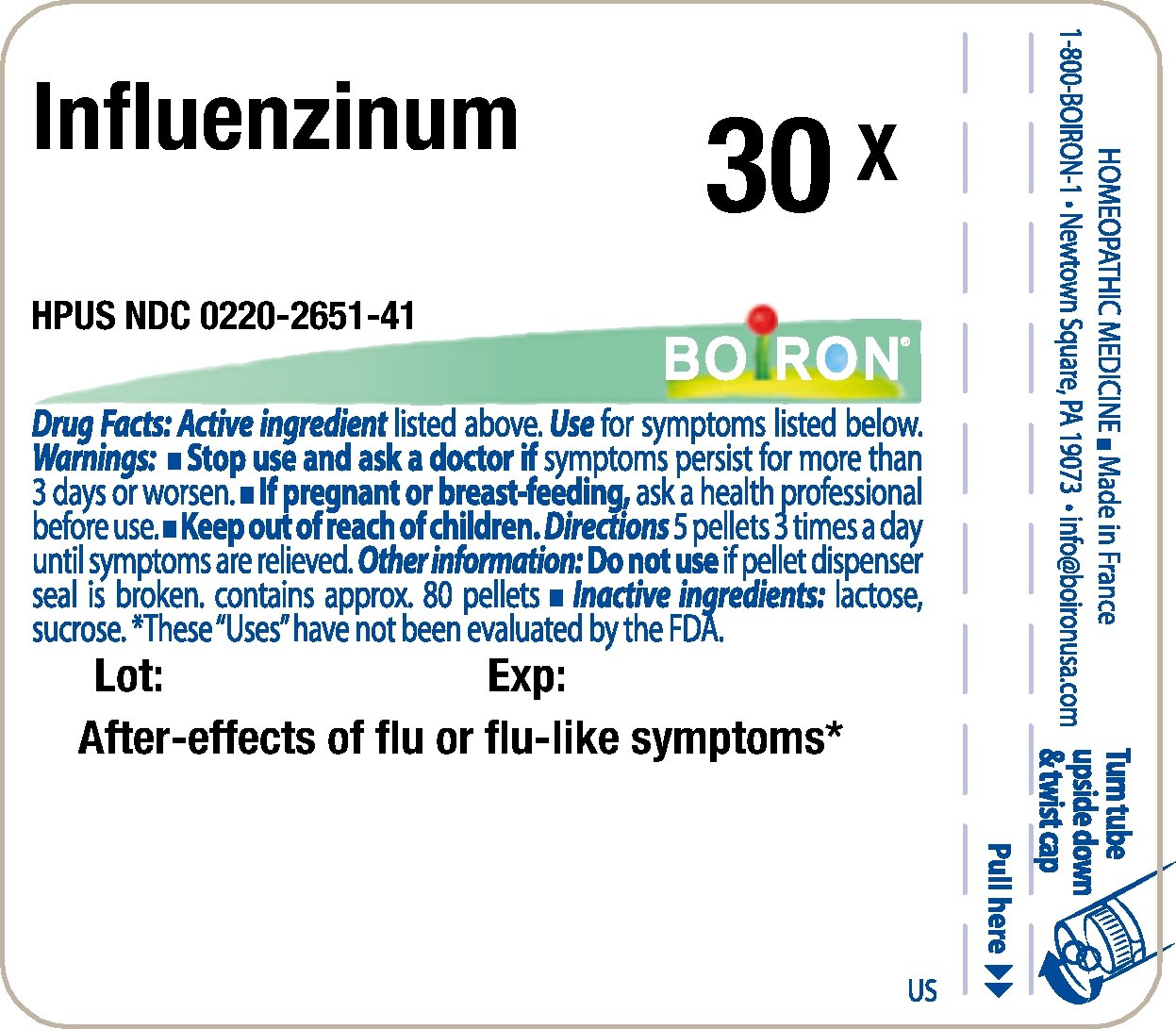
Principle Display Panel - Influenzinum HPUS 6C
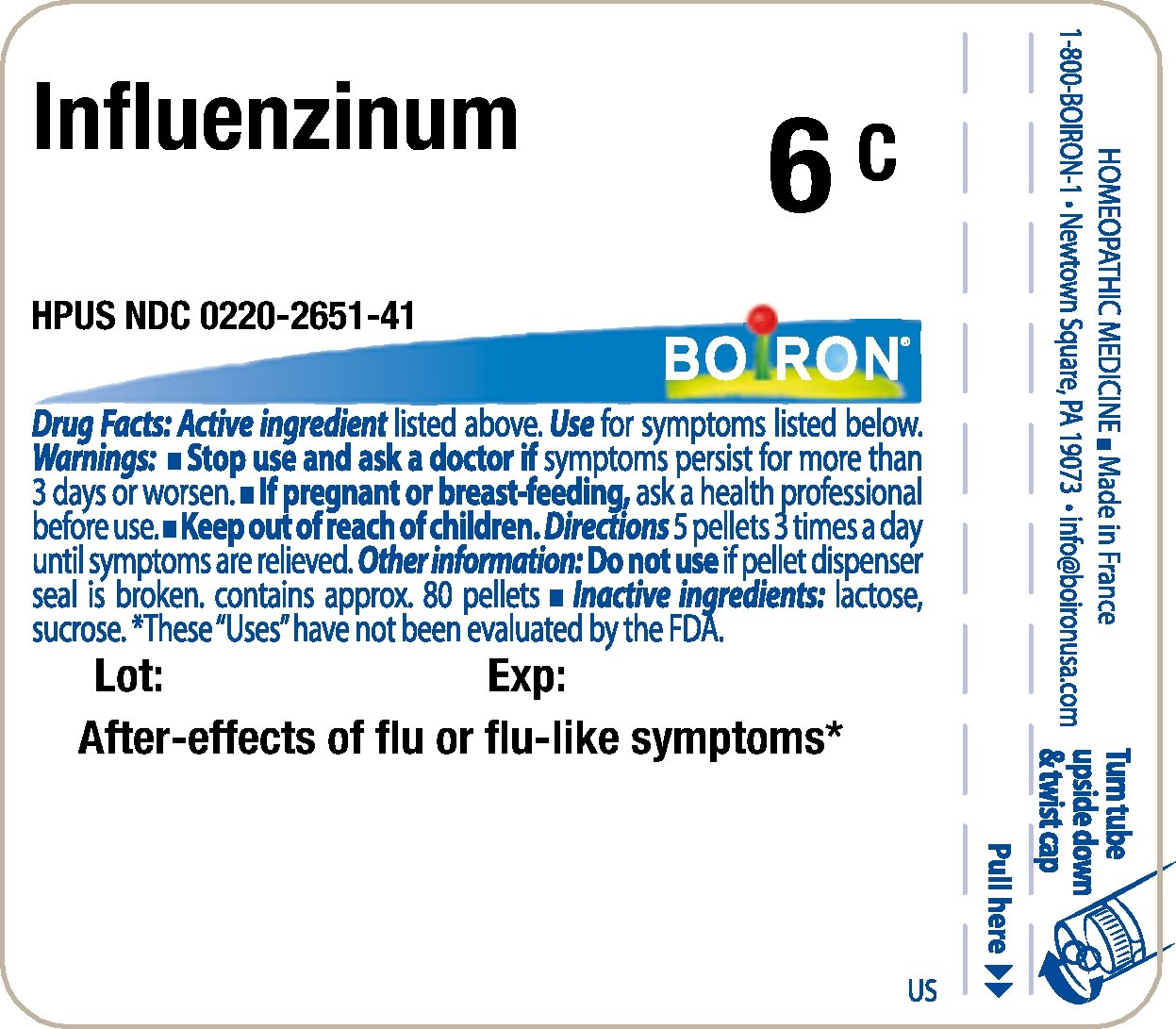
Principle Display Panel - Influenzinum HPUS 9C

Principle Display Panel - Influenzinum HPUS 12C

Principle Display Panel - Influenzinum HPUS 15C

Principle Display Panel - Influenzinum HPUS 30C

Principle Display Panel - Influenzinum HPUS 200CK
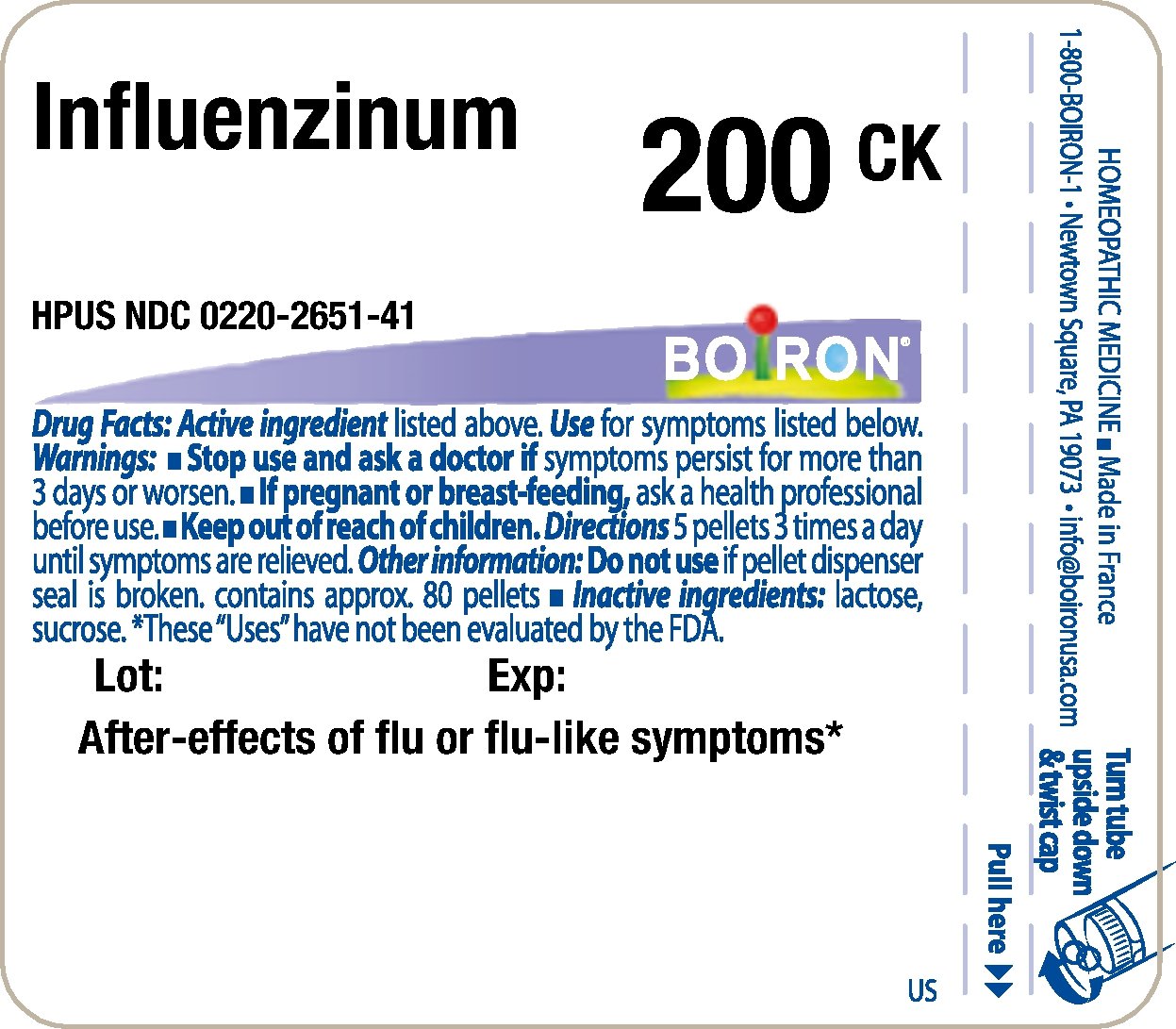
Principle Display Panel - Influenzinum HPUS 9C - Single Dose

INFLUENZINUM
influenza a virus a/michigan/45/2015 x-275 (h1n1) hemagglutinin antigen (formaldehyde inactivated), influenza a virus a/hong kong/4801/2014 x-263b (h3n2) hemagglutinin antigen (formaldehyde inactivated), influenza b virus b/brisbane/60/2008 hemagglutinin antigen (formaldehyde inactivated) pellet |
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Disclaimer









