Ful-Glo: Package Insert / Prescribing Info
Package insert / product label
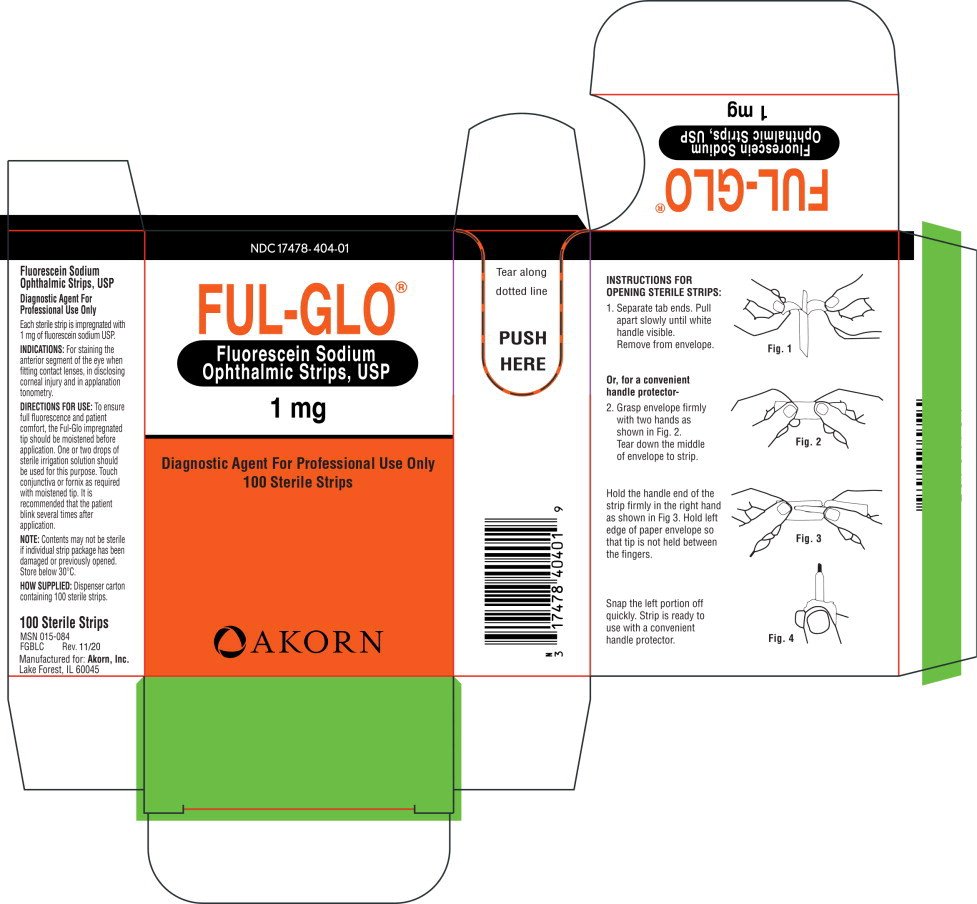
Generic name: fluorescein sodium
Dosage form: ophthalmic strip
Drug class: Ophthalmic diagnostic agents
Medically reviewed by Drugs.com. Last updated on Sep 9, 2024.
On This Page
Indications and Usage for Ful-Glo
For staining the anterior segment of the eye when fitting contact lenses, in disclosing corneal injury and in applanation tonometry.
Ful-Glo Dosage and Administration
To ensure full fluorescence and patient comfort, the Ful-Glo impregnated tip should be moistened before application. One or two drops of sterile irrigation solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
NOTE: Contents may not be sterile if individual strip package has been damaged or previously opened. Store below 30°C.
Related/similar drugs
How is Ful-Glo supplied
Dispenser carton containing 100 sterile strips.
100 Sterile Strips
MSN 015-084
FGBLC Rev. 11/10
Manufactured for: Akorn, Inc.
Lake Forest, IL 60045
Patient Counseling Information
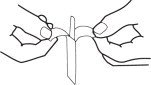
1. Separate tab ends. Pull apart slowly until white handle visible. Remove from envelope.
Or, for a convenient handle protector-
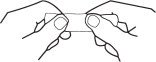
2. Grasp envelope firmly with two hands as shown in Fig. 2. Tear down the middle of envelope to strip.
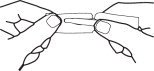
Hold the handle end of the strip firmly in the right hand as shown in Fig 3. Hold left edge of paper envelope so that tip is not held between the fingers.
Snap the left portion off quickly. Strip is ready to use with a convenient handle protector.
| FUL-GLO
fluorescein sodium strip |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akorn (117693100) |