Engystol: Package Insert / Prescribing Info
Package insert / product label
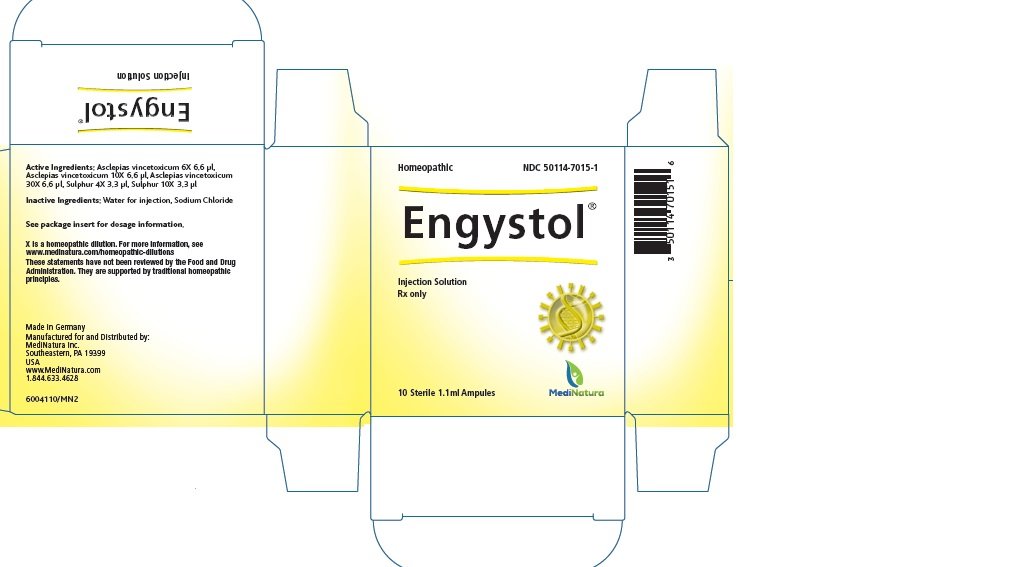
Generic name: cynanchum vincetoxicum root and sulfur
Dosage form: injection
On This Page
Engystol Description
|
Ingredient name |
Potency |
Quantity |
Final dilution |
|
Asclepias vincetoxicum |
6X |
6.6 μl |
8.22X |
|
Asclepias vincetoxicum |
10X |
6.6 μl |
12.22X |
|
Asclepias vincetoxicum |
30X |
6.6 μl |
32.22X |
|
Sulphur |
4X |
3.3 μl |
6.52X |
|
Sulphur |
10X |
3.3 μl |
12.52X |
Indications and Usage for Engystol
Engystol® Injection Solution is a homeopathic drug product indicated for the support of the immune system to reduce severity and duration of symptoms in viral infections, particularly in the early stages of colds and influenza-like illnesses.
Engystol Dosage and Administration
General Considerations
• The dosage schedules listed below can be used as a general guide for the administration of Engystol® Injection Solution.
• Engystol® Injection Solution may be administered s.c., i.d., i.m., or i.v.
• The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
• Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard any unused ampule contents.
• Draw up required dose into syringe.
• Discard any unused ampule contents. Do not reuse ampule.
-
Only licensed practitioners with sufficient expertise in injecting drugs, including the respective route of administration, should administer the product.
Standard Dosage:
Adults and children 12 years and older: 1 ml 1 to 3 times per 7 days.
Children 6 to 11 years: 0.7 ml 1 to 3 times per 7 days.
Children 2 to 5 years: 0.5 ml 1 to 3 times per 7 days.
Acute Dosage:
Adults and children 12 years and older: 1 ml daily, and then continue with standard dosage.
Children 6 to 11 years: 0.7 ml daily, and then continue with standard dosage.
Children 2 to 5 years: 0.5 ml daily, and then continue with standard dosage.
Contraindications
Engystol® Injection Solution is contraindicated in patients with known hypersensitivity to Engystol® or any of its ingredients.
Adverse Reactions/Side Effects
Post-marketing Experience
• The following adverse events have been identified during post-marketing use of Engystol® Injection Solution. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
• Allergic (hypersensitivity) skin reactions may occur in isolated cases.
To report SUSPECTED ADVERSE REACTIONS, contact MediNatura. at 1.844.633.4628 or info@medinatura.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Overdosage
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
Engystol - Clinical Pharmacology
Mechanism of Action
The exact mechanism of Engystol® Injection Solution is not fully understood.
Pharmacodynamics
Not applicable for homeopathic medicinal products.
| ENGYSTOL
cynanchum vincetoxicum root and sulfur injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - MediNatura (102783016) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hameln Pharma GmbH | 315869123 | manufacture(50114-7015) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biologische Heilmittel Heel | 315635359 | manufacture(50114-7015) | |